A Channel in the Heart of the Matter
How cells control the movement of ions, electrically charged species, in and out of the cell is a grand puzzle, whose completion will allow a thorough fundamental understanding of human physiology. A Danish-American team of researchers has found a piece of the puzzle with their determination of two structures of the human calcium-activated cation channel, TRPM4. Their results have been published in the journal <em>Science</em>.
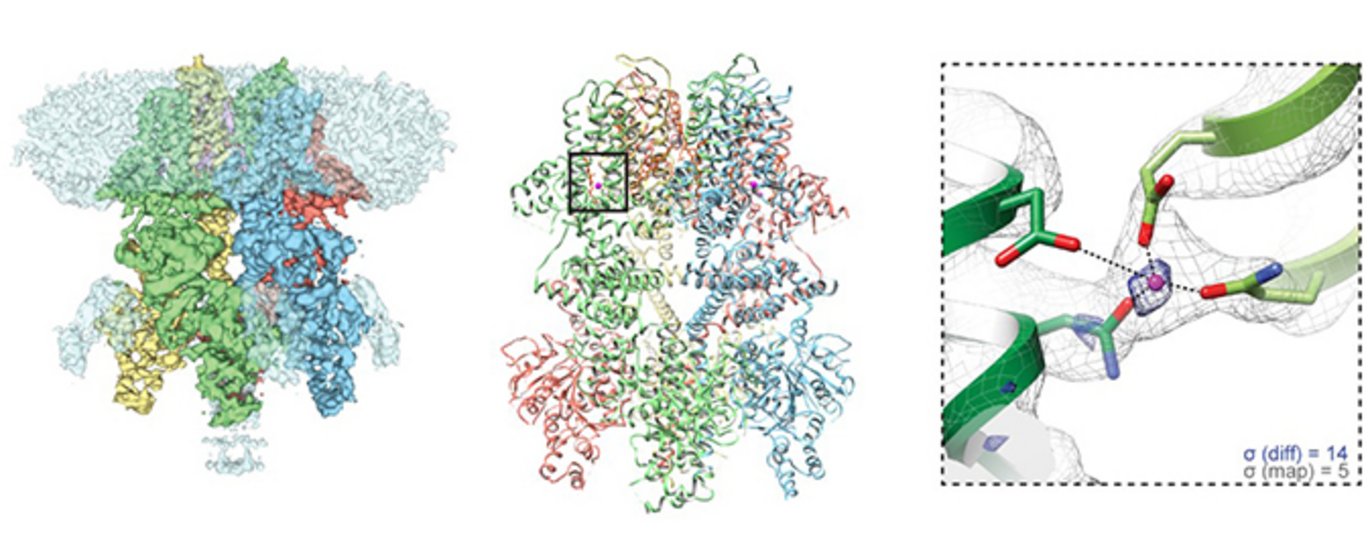
Thanks to a fellowship from the Danish Council of Independent Research, Postdoc Henriette Elisabeth Autzen from Aarhus University is currently stationed at the University of California, San Francisco. She and her American colleagues have now determined two structures of a human calcium-activated, cation channel, TRPM4. Last week, their results were published in the journal Science, and during the same week, another two structures on TRPM4 were published in Nature by two other research teams.
Henriette Autzen says, “We knew of one competitor, but the third study took us completely by surprise. However, as it turns out, all three studies complement each other beautifully, so in a single week, the field has gone from knowing very little about TRPM4 structure and function to having a much more in-depth understanding about modulation of TRPM4. Another surprise was the determination of a structure of TRPM8, which was published in the same issue of Science as TRPM4. As it turns out, the competition on getting the first structure of a protein belonging to the TRPM subfamily was tight.”
Structural biology has entered a new era
The transient receptor potential (TRP) cation channels compose a large, and functionally diverse, superfamily of integral membrane proteins that respond to a wide range of chemical and physical stimuli. The TRPM4 subtype is a calcium-activated, non-selective monovalent cation channel, widely expressed, but most abundantly in cells of the vascular system. While permeability to calcium is a defining feature for most TRP channels, TRPM4 is exclusively permeable to monovalent cations, but is activated by calcium and voltage. Mutations of the TRPM4 gene are linked to a variety of cardiovascular disorders while the healthy protein has significant impact on cell physiology through regulation of the membrane potential and calcium signaling.
The structures of human TRPM4 were determined using single particle electron cryo-microscopy (cryo-EM), a method which has gained high popularity among structural biologists since reaching near-atomic resolutions within recent years. The recent technological breakthroughs in single particle cryo-EM have enabled the technique to obtain unprecedented high resolution for a wide range of proteins, a feat that was acknowledged with the Nobel Prize in Chemistry this year.
In single particle cryo-EM, the purified protein is embedded in a thin film of vitreous ice at liquid nitrogen temperatures, preserving the native state of the protein. Images of many identical molecules adopting random orientations are recorded as 2D projections, which are in turn classified, aligned and averaged to generate a 3D reconstruction or density map analogous to an electron density maps determined by X-ray crystallography. The resolution of the 3D reconstruction is improved to near-atomic values by iteratively refining the geometric orientation parameters for each particle to a high accuracy, allowing de novo atomic model building by fitting the known protein sequence into the density map. Cryo-EM allows the protein to be reconstituted in a nano-sized disc of lipids, allowing the direct observation of how the protein interacts with lipids similar to its native environment, which is also the case for TRPM4.
Visualizing the target
The two TRPM4 structures determined by Postdoctoral Fellow Henriette Autzen and colleagues were determined with and without calcium bound. Comparison of the two structures revealed a calcium binding site in the transmembrane domain, and conformational changes associated with ion-binding. The structures provide a first glimpse into how calcium may prime this and other calcium-dependent TRPM ion channels for voltage-dependent opening.
Henriette adds, “We knew from the literature that there had to be a binding site for calcium somewhere in TRPM4, but no one knew where it was located. The structures are the first steps towards understanding TRPM4 and other related TRPM channels on a functional level in health and disease.”
The structures of TRPM4 allow researchers to map the location of mutations associated with cardiac diseases, in addition to designing new drugs targeting TRPM4 in treatment of these diseases. The structures can also be used for predicting what other related TRPM channels look like.
The Danish-American team of researchers are planning to continue their work on TRPM4. “We would like to know why TRPM4 is one of the only TRP channels that are selectively permeable to monovalent ions, and how the channel is modulated by certain types of lipids,” Henriette Autzen concludes.
Henriette Autzen is a postdoctoral fellow at the Department of Molecular Biology and Genetics at Aarhus University with an individual postdoc grant from the Danish Council for Independent Research, conducting her research in the laboratory of Professor Yifan Cheng at University of California, San Francisco, a pioneer in the field of single-particle cryo-EM on membrane proteins.The project was a collaboration with Professor David Julius.
The study “Structure of the human TRPM4 ion channel in a lipid nanodisc” was published in the journal Science.
Link to the two other competing articles in Nature:
- Structures of the calcium-activated, non-selective cation channel TRPM4
- Electron cryo-microscopy structure of a human TRPM4 channel
Link to the same issue of Science about TRPM8:
For further information, please contact
Dr. Henriette Autzen
henriette@mbg.au.dk
Department of Molecular Biology and Genetics, Aarhus University, Denmark
Currently:
Department of Biochemistry and Biophysics, Genentech Hall
University of California, San Francisco, California, USA
