Scientists map type 2 diabetes’ stages of evolution
Many neurodegenerative diseases, e.g. Parkinson, Alzheimer, and Huntington, are caused by the formation of fibrils that is developed from tiny twisted proteins. As a rule one specific protein is connected to one specific disease. However, new research suggests that the formation of fibrils can be associated with other diseases, e.g. type 2 diabetes. Scientists at iNANO have by means of Atomic Forced Microscopy (AFM) shown how fibrils are formed and which stages the formation undergoes. The study was published in <em>PNAS</em>
Match-up: old age versus genetics/modern way of life
For decades scientists thought that type 2 diabetes was related to old age. However, a growing number of young people are diagnosed with type 2 diabetes every day. During the period 2001 to 2011 the number of children and teenagers who were diagnosed with diabetes increased with 79%. The disease is characterised by the fact that cells become less sensitive towards insulin. Hence, insulin is an important peptide hormone that regulates the metabolism of sugar within the body.
The absorption of sugar increases when insulin connects itself to the receptors which are located at every cell surface. The concentration of sugar in the blood increases as the cells’ sensitivity towards insulin decreases. The disease can have a number of different symptoms, e.g. sugar in the urine, thirst, nausea, and fatigue.
Diabetes is mainly hereditary, however, it may also be a product of the environment. The unfavourable development regarding the population’s eating habits, overweight problems and lack of regular exercise plays a central role in connection with developing type 2 diabetes. According to Diabetisforeningen (the Danish diabetic association) more than 245.000 Danes have been diagnosed with type 2 diabetes. Moreover, it is estimated that a similar number live with the disease without knowing it.
Is the villain a tiny protein?
Additionally, type 2 diabetes can be a product of the formation of fibrils in the pancreas cells (Westemark, Andersson et al. 2011). These cells produce insulin and control the body’s insulin level. Given that most patients diagnosed with type 2 diabetes have fibril formation in the pancreas cells, several studies were carried out in the 1980s.The goal was to determine what these fibrils consist of (Cooper, Willis et al. 1987).
As a rule the source is a single tiny protein. This applies to both type 2 diabetes and neurodegenerative diseases (Westemark, Andersson et al. 2011). The function of the tiny protein was originally unknown, however, now it is known that the protein is stored with insulin in the vesicles that carries insulin from the pancreas cells to the blood stream.
Fluorescence can help detect the formation of fibril
A detail understanding of how fibrils are formed is important in connection with the manufacturing of medicine.
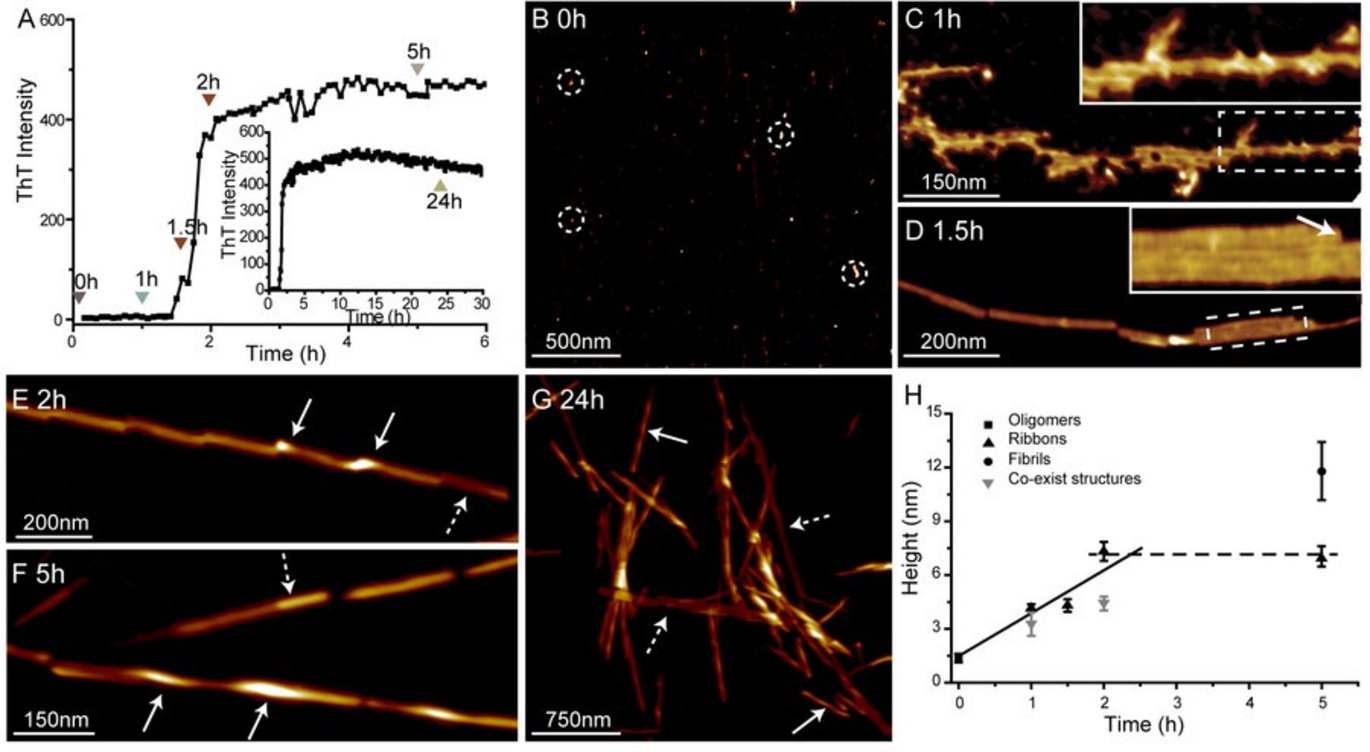
The researchers investigated the fibril formation of the part of hIAPP, between the 20th and 29th aminoacid. This part is considered to be the core of the fibril formation process. The results indicate that the formation of fibril is depended on the formation of fibril seeds.
In the begging of the experiment (until point 0 (fig. 1B)) only tiny particles which measures approximately 1.4 nm are visible. Hence, there is no fixed structure. After 60 minutes a messy structure with a lot of branches is visible. After 90 minutes a growing organised structure is visible (fig. 1D).
Subsequently, the amyloid strings begin to twist and transform into amyloid fibrils which results in that fact that the fibrils become taller than the amyloid strings. The point where the twisted fibrils are taller is shown as shining spots (highlighted by white arrows). However, it is not the whole string which transforms into a fibril. Hence, the two stages co-exist (highlighted by striped arrows).
Moreover, after 24 hours (fig. 1G) some strings have not been transformed into fibrils (highlighted by striped arrows). Based on fig. 1H it is possible to form a picture of fibril formation: the connection process starts within the first 1-1,5 hours. During the following 30 minutes the strings which measures 4,3 nm are formed after which they starts to become thicker, cf. until they are 7,3 nm wide.
Scientific article published in the journal PNAS: Coexistence of ribbon and helical fibrils originating from hIAPP20–29 revealed by quantitative nanomechanical atomic force microscopy.
The research highlight in Nature Chemistry
More information
 | Department of Molecular Biology and Genetics / iNANO | |
