Nanorobot for transporting drugs in the body
The first step has been taken towards developing a nanorobot that – in the long run – will enable the targeted transport of medications in the body.

A nanorobot is a popular term for molecules with a unique property that enables them to be programmed to carry out a specific task. In collaboration with colleagues in Italy and the USA, researchers at Aarhus University have now taken a major step towards building the first nanorobot of DNA molecules that can encapsulate and release active biomolecules.
In time, the nanorobot (also called a DNA nanocage) will no doubt be used to transport medications around in the body and thereby have a targeted effect on diseased cells.
Design using the body’s natural molecules
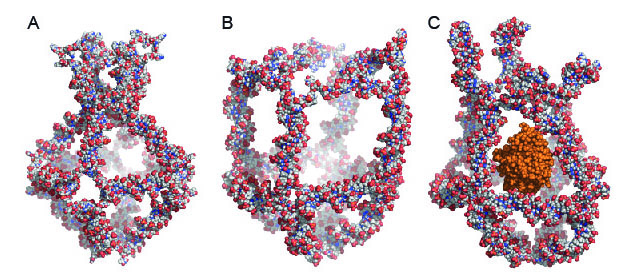
Using DNA self-assembly, the researchers designed eight unique DNA molecules from the body’s own natural molecules. When these molecules are mixed together, they spontaneously aggregate in a usable form – the nanocage (see figure).
The nanocage has four functional elements that transform themselves in response to changes in the surrounding temperature. These transformations either close (figure 1A) or open (figure 1B) the nanocage. By exploiting the temperature changes in the surroundings, the researchers trapped an active enzyme called horseradish peroxidase (HRP) in the nanocage (figure 1C). They used HRP as a model because its activity is easy to trace.
This is possible because the nanocage’s outer lattice has apertures with a smaller diameter than the central spherical cavity. This structure makes it possible to encapsulate enzymes or other molecules that are larger than the apertures in the lattice, but smaller than the central cavity.
The researchers have just published these results in the renowned journal ACS Nano. Here the researchers show how they can utilise temperature changes to open the nanocage and allow HRP to be encapsulated before it closes again.
They also show that HRP retains its enzyme activity inside the nanocage and converts substrate molecules that are small enough to penetrate the nanocage to products inside.
The encapsulation of HRP in the nanocage is reversible, in such a way that the nanocage is capable of releasing the HRP once more in reaction to temperature changes. The researchers also show that the DNA nanocage – with its enzyme load – can be taken up by cells in culture.
Looking towards the future, the concept behind this nanocage is expected to be used for drug delivery, i.e. as a means of transport for medicine that can target diseased cells in the body in order to achieve a more rapid and more beneficial effect.
The research was carried out at the Department of Molecular Biology and Genetics and the Interdisciplinary Nanoscience Centre (iNANO), Aarhus University, in collaboration with researchers from Duke University (USA) and the University of Rome (Italy).
For more information, please contact
Associate Professor Birgitta R. Knudsen
Department of Molecular Biology and Genetics/Interdisciplinary Nanoscience Centre
Aarhus University
brk@mb.au.dk – mobile: +45 6020 2673
Postdoctoral Fellow Sissel Juul
Department of Biomedical Engineering
Duke University, Durham, North Carolina, USA
Sissel.juul@duke.edu – mobile: +1 919 323 2291
