New study challenges our understanding of the immune system
Researchers have created a radical new view of how immune cells recognise threats such as viruses. The discovery could be used to design better vaccines and to gain a deeper insight into autoimmune diseases and allergies.
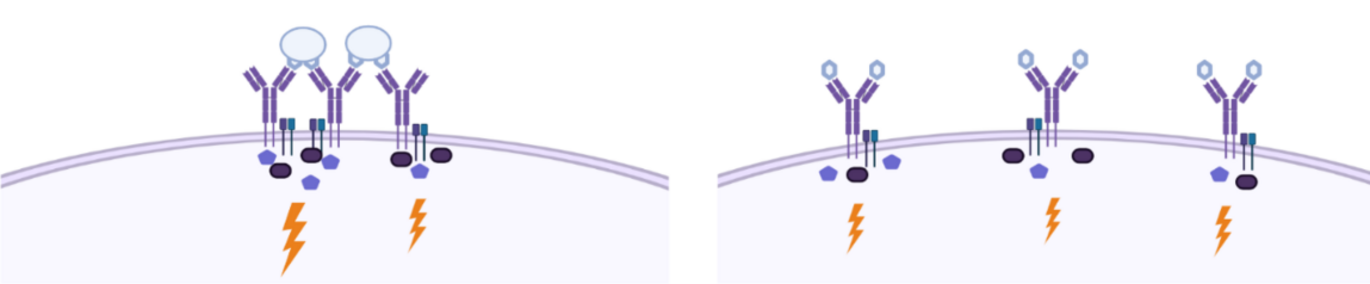
A recently published study from Aarhus University may mean a textbook chapter on the immune system will have to be rewritten.
In the study, published in the journal Nature Communications, the researchers reveal crucial new knowledge about B cells, which form a vital element in the body’s defence system. B cells are the cells that generate protective antibodies when we are vaccinated or have an infection – and it is also the B cells that produce harmful antibodies in connection with allergies or autoimmune diseases.
The researchers have examined the earliest step in activating the B cells, namely the activation mechanism that is triggered when the cells recognise a specific target or ‘enemy’ – an antigen.
“Previously, it was believed that the antigens from, for example, viruses or vaccines would have to cross-bind a B-cell’s receptors on the cell surface (see illustration). That’s what it says in all the textbooks. But now we have shown that even antigens that can only bind one receptor at a time are able to activate the B cells,” says Søren Degn, associate professor at Department of Biomedicine, who is the senior author of the article.
Method
The researchers applied an interdisciplinary approach, which encompassed, amongst other things, a ground-breaking super-resolution microscopy technique called DNA PAINT, as well as a unique nanoscaffold made of modified nucleic acids and two sophisticated, genetically modified mouse DNA strains.
The discovery is important on several levels, he explains.
“The result is significant because it represents a breakthrough in our understanding of how these important immune cells ‘recognise’ their enemies. Once we understand what is going on, we can imitate it in the design of new vaccines, to ensure maximum effect. One might say that our findings can make us better at mimicking the pathogenic microorganisms, and thus better at provoking or ‘cheating’ the immune system into generating a good immune response when we vaccinate.”
A hotly debated topic in the field
The discovery is interesting for both the immunological field and for cell biology in general, because the researchers have shed new light on the foundation for how receptors on the surface of cells send signals into the cells – a key biological process.
“The study enables us to better understand the background for one of the most important processes in the immune system, and one of the most important processes in cell biology. But it is clear that, in the long term, this could also have important application-oriented aspects,” says Søren Degn.
The researchers have begun preclinical vaccine trials with the aim of translating the findings into clinically relevant vaccine design. They are also attempting to use the same tools in reverse, to target and turn off harmful immune system responses such as allergic reactions and autoimmune diseases.
“When we understand how the B cells are activated, we can create better vaccines. In the slightly longer term, we may also be able to switch off B-cell activation in cases where it is harmful. We are studying both of these in the CellPAT basic research centre at Aarhus University,” says Søren Degn.
For many years, the activation of B cells has been the object of a great deal of discussion among researchers, because the predominant model for how immune recognition takes place could not explain all of the observations.
In the new study, the researchers at the Department of Biomedicine and iNANO in Aarhus, in a cross-disciplinary collaboration with the Max Planck Institute in Munich, have created new tools that make it possible to puncture the predominant model and thereby bury the decades-old paradigm.
“We have shown that the way in which the activation of B cells has been explained over the past thirty or forty years is wrong. This is an important finding, because it opens the door to better vaccines and better treatment of a large group of diseases,” says Søren Degn.
Behind the research results
- The study consists of ex vivo cell experiments, i.e., in vitro studies of cells from mouse models, nanotechnology and super-resolution microscopy (advanced microscopy).
- The study is the result of a collaboration between several groups in the Department of Biomedicine at Aarhus University (Degn, Thiel and Vorup-Jensen), iNANO (Kjems) and the Max Planck Institute in Munich (Jungmann). The work stems from the basic research centre CellPAT, the Centre for Cellular Signal Patterns.
- External funding (main funders only): The Danish National Research Foundation and the Carlsberg Foundation
- Learn more: Antigen footprint governs activation of the B cell receptor | Nature Communications
Contact
Associate Professor Søren Egedal Degn
Aarhus University, Department of Biomedicine
sdegn@biomed.au.dk
Mobile: +45 2214 1703
Professor Jørgen Kjems
Department of Molecular Biology and Genetics
Interdisciplinært Nanoscience Center
Aarhus University
jk@mbg.au.dk - Mobile: +45 2899 2086
