Researchers have determined the three-dimensional atomic structure of a protein important for organ functions
The so-called NKCC1 protein is found throughout the human body where it has important functions in organs that process fluids or perform neurotransmission. A Danish research team has now determined a three-dimensional atomic structure of the NKCC1 protein, which supports future work to identify novel compounds that interfere with NKCC1 function. Such compounds may help in, for example, kidney and brain disorders.
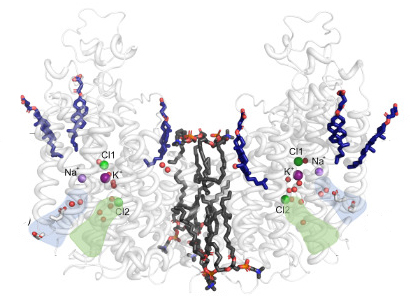
NKCC1 is a human chloride transporter that has the ability to transport sodium, potassium, and chloride from the exterior into cells. In the kidney, for example, NKCC1 type proteins ensure that these ions are reabsorbed from the urine, and generally NKCC1 is important for osmotic cell volume regulation. In brain, NKCC1 and related proteins are important for chloride gradients that are vital for the electrical signaling in neuronal networks.
Using cryo-electron microscopy (cryo-EM), a team from Poul Nissen’s laboratory with colleagues from the Fenton and Hartmann laboratories at Aarhus University (AU) and the Lindorff-Larsen laboratory at University of Copenhagen (KU) have determined the three-dimensional atomic structure of NKCC1 (a so-called Na+- K+- 2 Cl– cotransporter) and investigated its function.
Insights into the three-dimensional atomic structure and dynamics of NKCC1 including the bound ions, lipids and water molecules as well as ion transport studies in cells provide important new information on NKCC1 function, which is driven by the sodium gradient established by the sodium-potassium pump.
The studies that the team present in an article in EMBO Journal reveal a surprising mechanism for release of the ions into the cell that begins with one of the two bound chloride ions, and only then the sodium ion and finally the other chloride and the potassium ion. The researchers will now continue to try to identify novel compounds that interfere with NKCC1 function and that may help in for example kidney and brain disorders.
The project was highly challenging from the very beginning and was started almost 10 years ago as a collaboration between the Nissen and Fenton laboratories. First-author and PhD student Caroline Neumann (now graduated) and colleagues from the Nissen laboratory undertook important collaborations with Prof. Rune Hartman’s laboratory (AU) to establish an advanced expression system for efficient protein production, and with Prof. Robert Fenton’s laboratory (Dept. of Biomedicine, AU) for functional studies of the transporter in mammalian cells. Finally, a collaboration was initiated with Prof. Kresten Lindorff-Larsen’s group from the University of Copenhagen for the computational simulations of the NKCC1 dynamics and ion release.
SUPPLEMENTARY INFORMATION, INCLUDING CONTACT INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
Study type | Experiment |
External funding | This work has been supported by the Lundbeck Foundation with a PhD stipend to Caroline Neumann and project financing through the BRAINSTRUC Center and a Professorship grant (to Poul Nissen). Support has also been received from the Novo Nordisk Foundation and the Danish Council for Independent Research (DFF). |
Conflicts of interest | The researchers declare that there are no conflicts of interest. |
Link to scientific paper | Caroline Neumann, Lena Lindtoft Rosenbæk, Rasmus Kock Flygaard, Michael Habeck, Jesper Lykkegaard Karlsen, Yong Wang, Kresten Lindorff-Larsen, Hans Henrik Gad, Rune Hartmann, Joseph Lyons, Robert A. Fenton, Poul Nissen Cryo-EM structure of the human NKCC1 transporter reveals mechanisms of ion coupling and specificity EMBO Journal |
Contact information | Postdoc Caroline Neumann – e-mail: caroline@mbg.au.dk Professor Poul Nissen - Email: pn@mbg.au.dk. Phone: +4528992295 |
