Researches develop new protein for prevention of influenza virus infection
An international research team has developed a new protein drug which has the potential to be used for protection against all types of influenza infection. By delivering the drug as a DNA vector it may also function as a universal influenza vaccine.
Each year, infection with influenza virus is estimated to cause severe illness in 3-5 million people and more than 290,000 deaths, globally. Humans are infected by three types of influenza virus (A, B and C), but most commonly with type A or type B. Within influenza A and B, there are many thousand different strains, which are classified into subtypes based on the surface proteins neuraminidase and hemagglutinin (HA). For influenza A, there are 18 different subtypes of HA (H1-18), while influenza B has two different lineages.
The most effective way to prevent an infection is by vaccination. With a traditional influenza vaccine, the immune system will generate antibodies against HA, which can neutralize the virus and prevent infection. However, as the gene coding for HA is mutated upon replication of the virus, the antibodies generated following vaccination may not neutralize the virus if the surface of HA has changed. Thus, the vaccine will only work against the same or closely related strains and has to be renewed each time a new strain will dominate. The choice of what stains to include in the vaccine is based on predictions on which type of virus that will circulate in next season, and if the predications are wrong, the vaccine may have limited effect.
Identification of antibodies that can neutralize different types of virus
In an attempt to find a better vaccine and treatment options, the researchers previously identified broadly neutralizing antibodies which recognizes conserved regions on HA and are able to neutralize a broad range of different influenza virus subtypes. However, these antibodies cannot directly be used for prevention of infection as this would require multiple injections of a mixture of antibodies during the entire influenza season to protect against both influenza A and B and to obtain a sufficient concentration of antibodies.
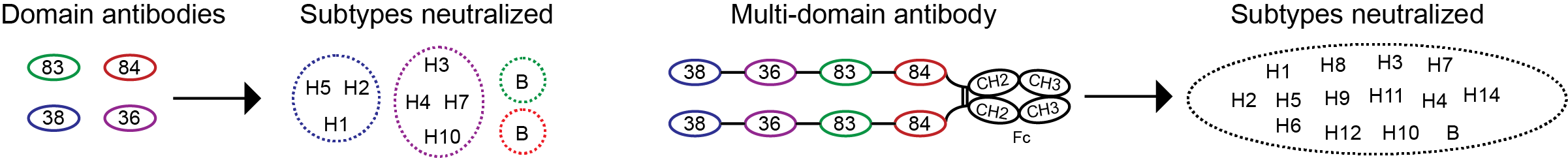
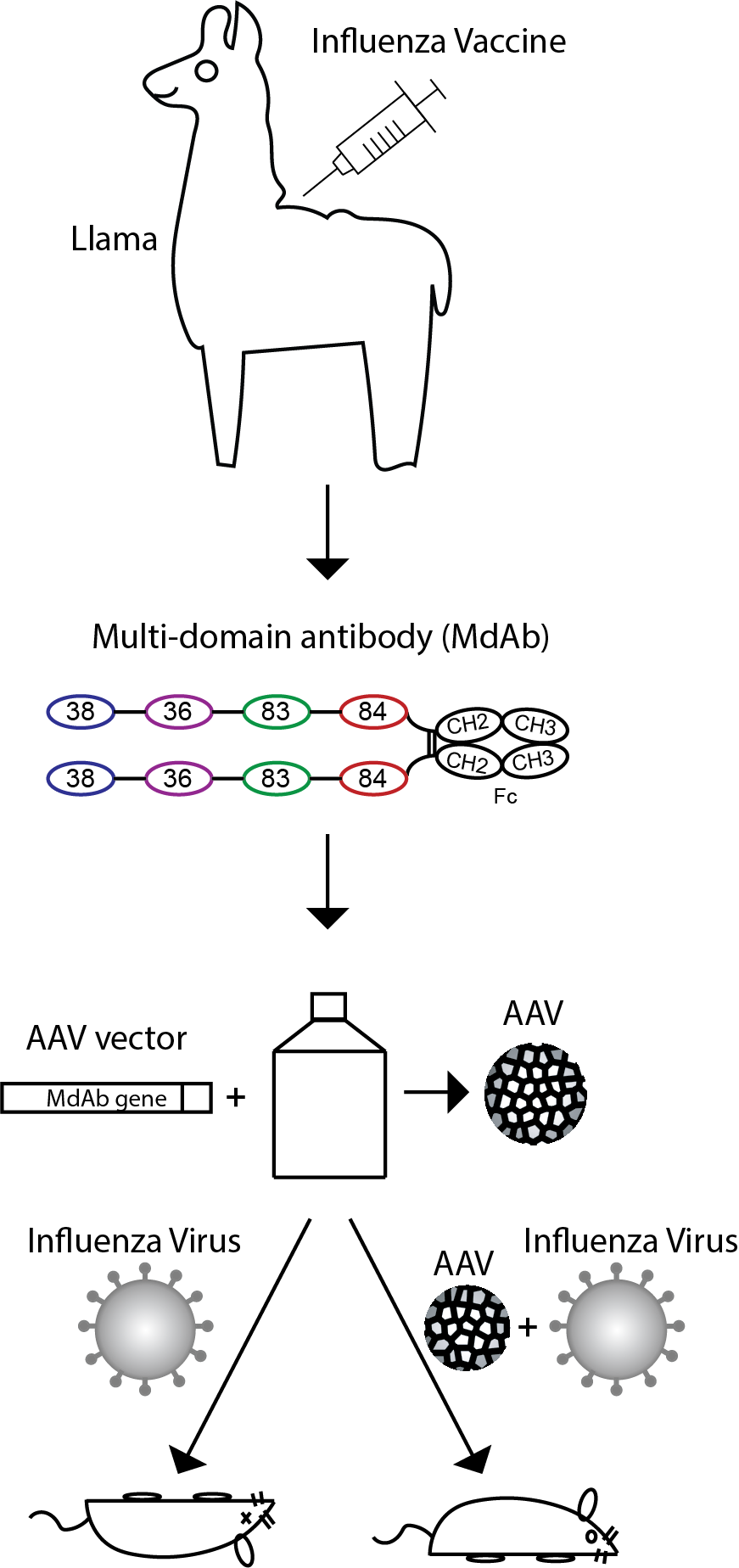
To overcome these limitations, the researchers used an alternative strategy using domain antibodies isolated from llamas immunized with an influenza vaccine and an HA protein.
- First a number of domain antibodies that were able to broadly neutralize viruses were isolated, explains Assistant Professor Nick Stub Laursen. The atomic structure of HA in complex with the domain antibodies were determined and showed that all recognized conserved areas on the HA protein, which explains how the domains antibodies can recognize so many different subtypes of influenza, adds Nick Laursen.
- To generate a protein that would neutralize both influenza A and influenza B, four different domain antibodies were fused together with peptide linkers to generate a multi-domain antibody, continues Nick Laursen. The resultant protein was much more potent in neutralizing influenza virus and was also able to neutralize both influenza A and B subtypes. Next, the four-domain protein was fused to an immunoglobulin Fc fragment and tested in an in vivo model where it showed protection against infection with influenza A and influenza B virus, concludes Nick Laursen.
To avoid continuous administration of the protein, which would be necessary to provide protection during the entire influenza season, the researchers engineered the DNA coding for the protein into an adeno-associated virus (AAV) such that the protein would be expressed locally upon delivery of the AAV. When the AAV was delivered through the nose, it protected against infection by influenza A and influenza B virus. The researchers suggest that if the results from mice can be translated into humans, this strategy could be used in the future as a universal vaccine against influenza and other highly variant viruses.
The results were published online this week in the international journal Science:
”Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin”
Nick S. Laursen, Robert H. E. Friesen, Xueyong Zhu, Mandy Jongeneelen et al.
DOI: 10.1126/science.aaq0620
For further information, please contact
Assistant Professor Nick Stub Laursen
Department of Molecular Biology and Genetics, Aarhus University
nsl@mbg.au.dk - +45 30497099
