Well-known RNA molecule is not present in cancer cells after all
A so-called circular RNA molecule, which is thought to be carcinogenic, is not present in cancer cells after all. A Danish research team has published the new results in <em>Nature Communications</em>.
Cancer has traditionally been seen as a genetic disease caused by mutations in important genes, but it has gradually become clearer that many other types of molecular changes contribute to the onset and development of cancer – so-called epigenetic changes. This can, for example, be chemical modifications at the DNA level that affect how much protein product a given gene gives rise to, without changing the DNA sequence of the gene itself, but also through so-called microRNA molecules that work by – post-transcriptionally – regulating genes activity.
In recent years, a new group of biomolecules has been discovered, the so-called circular RNA molecules, some of which have been found to bind to and inhibit microRNA molecules, thereby adding another layer to the complex regulation of human genes.
Recently, we have witnessed an explosion of research activity within this new research area, but a Danish research team shows in a new study published in Nature Communications that many of the first studies of circular RNA in cancer have probably drawn hasty conclusions, which is primarily due to a lack of spatial analyses.
Cancer should be seen as a complex interplay between cancer cells and benign cells
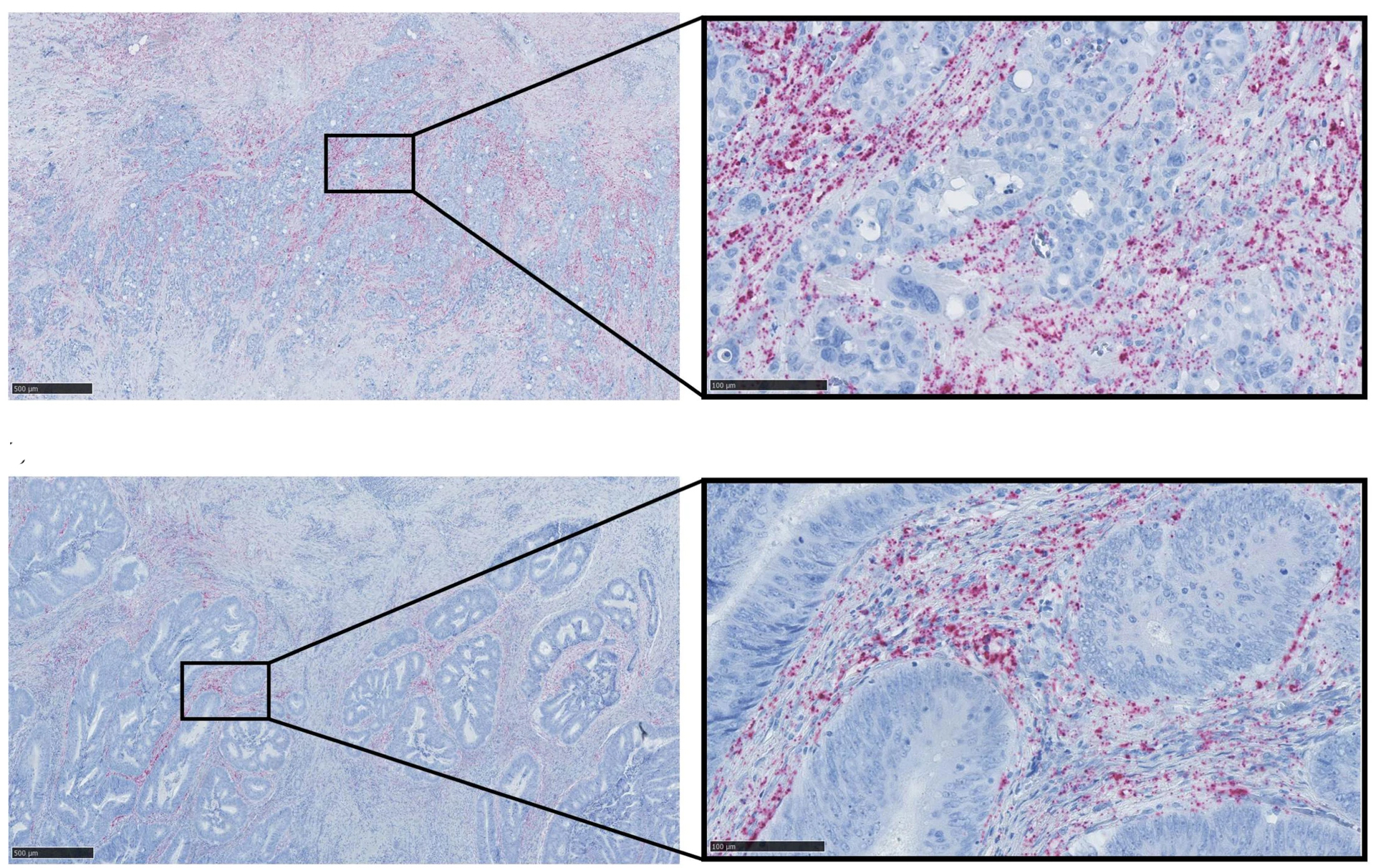
Thus, we see that an otherwise very well-described circular RNA molecule, called ciRS-7, is in fact not present in the cancer cells at all in a number of frequently occurring cancers, despite being described as a carcinogenic molecule that was supposed to act by binding a cancer-inhibiting microRNA, called miR-7, in the cancer cells.
Instead, we see that other benign cell types in a malignant tumour express very high levels of ciRS-7, suggesting that it may instead contribute to cancer growth through the so-called microenvironment found in a malignant tumour.
Lasse Sommer Kristensen: "Our results clearly demonstrate how important it is to think of cancer as a complex interplay between cancer cells and the many other types of benign cell types found in a malignant tumour when trying to understand the molecular mechanisms that contribute to cancer growth.”
In other words, the classic cancer cell lines grown in the laboratory – and often used when trying to understand new molecular mechanisms – may not always give us a true picture of what is at stake in an actual malignant tumour.
In addition, the results of the Danish researchers exemplify how spatial analyses of malignant tumours from patients can contribute with important knowledge that would not have been gained by – in the traditional way – analysing all DNA or RNA from a malignant tumour (both derived from cancer cells and benign cells) in a pooled assay.
The article was published in Nature Communications.
https://doi.org/10.1038/s41467-020-18355-2
For further information, please contact
Associate Professor Lasse Sommer Kristensen
Formerly: Institut for Molekylærbiologi og Genetik/iNANO
Nu: Institut for Biomedicin, Aarhus Universitet
lasse@biomed.au.dk
Professor Jørgen Kjems
Department of Molecular Biology and Genetics (MBG)
Interdisciplinary Nanoscience Center (iNANO)
Aarhus University, Denmark
+45 28992086 - jk@mbg.au.dk
Clinical Associate Professor Henrik Hager
Department of Clinical Pathology
Lillebælt Hospital, Denmark
Henrik.Hager@rsyd.dk
