Danish researchers release ground-breaking knowledge about calcium pumps in cells
When animals and plants are exposed to influences such as bacterial attack, odour and cold, calcium ions flow into the cells. The calcium provides the cells with a signal about what is going on outside, but as high concentrations of calcium are toxic to the cells, it must be quickly pumped out again. Researchers from the Danish National Research Foundation’s PUMPkin Centre at both the University of Copenhagen and Aarhus University have now shown that calcium pumps in the cell’s outer membrane adjust the pump speed very accurately to the calcium concentration. These findings have just been published in Nature.

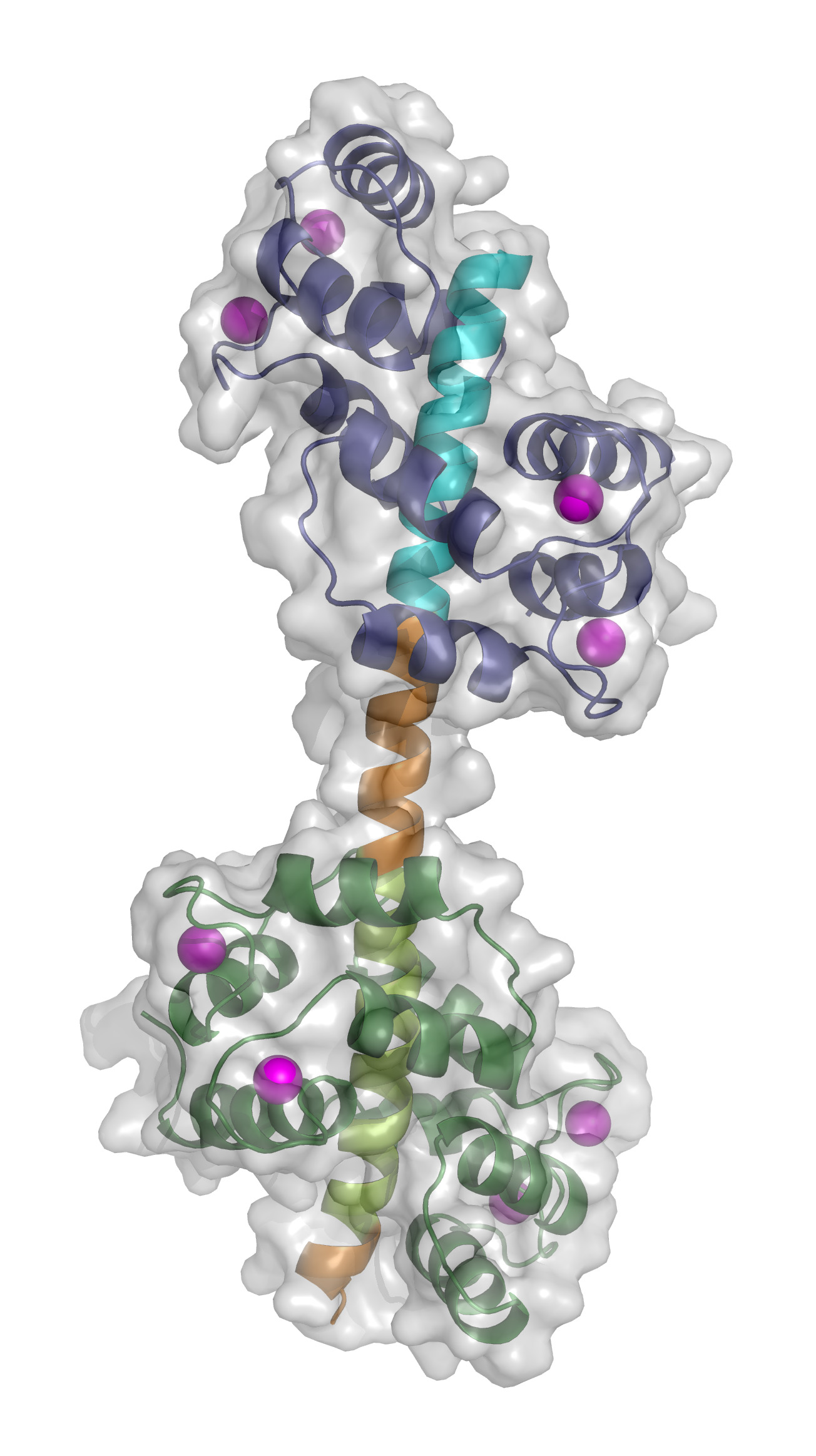

The calcium pump is located in the thin membrane that surrounds the cells of humans, animals and plants. Leading researchers from Aarhus University and the University of Copenhagen have now provided new information of how the calcium pump regulates the amount of calcium in the cells. This amount is critical to the health and survival of the cell.
- “It turns out that the calcium pump can accurately measure the cell’s calcium content and adjust its speed in accordance with this information. This prevents the concentration of calcium ions in the cytoplasm from reaching a critical concentration that damages the cells. The calcium pump is inactive when the concentration of calcium is low, but it is activated stepwise when the calcium concentration increases,” say Postdoctoral Fellows Henning Tidow and Lisbeth Rosager Poulsen, who took part in the joint research project.
The researchers’ starting point was the calcium pump located in the cell membrane of the model plant thale cress (Arabidopsis thaliana), but the regulatory mechanism also applies to the corresponding calcium pump in humans and animals.
Calcium pumps bind two calmodulin proteins
Previous studies have shown that calcium pumps in both animals and plants work together with a protein called calmodulin. When there are many calcium ions in a cell, some of these bind to calmodulin, which is thereby able to activate the calcium pump.
- “We purified the part of the calcium pump that interacts with calcium-activated calmodulin, and we managed to crystallise a protein complex. To our great surprise, we found that the calcium pump binds two calmodulin proteins, and not just one as always assumed,” explains Dr Tidow.
Calcium pump with three-step regulation
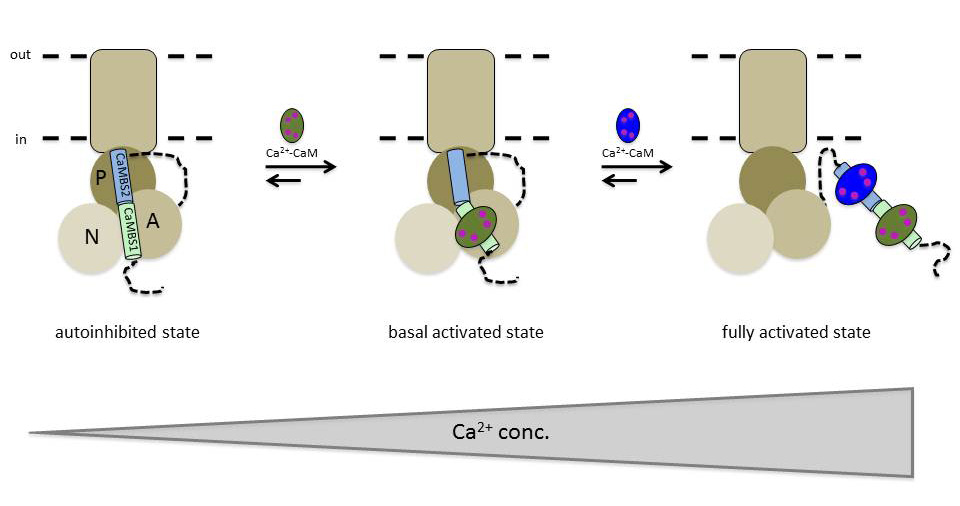
The fact that two calmodulin proteins are involved in the regulation of the calcium pump activity means that the calcium pump has three steps. It is switched off when no calcium-activated calmodulin is bound, it pumps at medium speed when binding occurs at one calmodulin protein, and it pumps at full speed when both calmodulin proteins are bound.
- “Calcium pumps need considerable energy to transport calcium out of the cell. It is therefore important that they are only activated when there is a need to remove calcium. With two calmodulin-binding domains in the calcium pump, the cell can adjust the transportation to be energy efficient, at the same time as being able to quickly reduce the number of calcium ions if the concentration approaches a toxic level,” Dr Poulsen concludes.
Mathematics reveals biological function
The researchers also used mathematical network modelling to further identify whether the calcium pump works differently depending on whether it is activated by zero, one or two calmodulin proteins. This revealed another characteristic of calcium pump regulation of calcium in the cell.
- “We could show that the cell only responded to incoming calcium when concentrations above carefully defined threshold values were found. This may be important for the way cells define their status in the circadian rhythm or during cell division, for example,” concludes Dr Tidow.
The results have just been published in the prestigious international journal Nature, and they may form the basis for the development of new drugs and new methods of food production.
Unique interdisciplinary collaboration
The research project was carried out as a unique interdisciplinary collaboration – in disciplines such as bioinformatics, protein crystallography, biophysics, enzyme kinetics, cell biology and particularly mathematical network modelling – between researchers from Aarhus University and the University of Copenhagen at the Centre for Membrane Pumps in Cells and Disease (PUMPkin) – one of the Danish National Research Foundation’s Centres of Excellence – www.PUMPkin.au.dk
Facts
Long-standing collaboration
The scientists from Aarhus University and the University of Copenhagen are world renowned for their research into both crystal structures and the biochemical and biological characterisation of ion pumps. This is not the first time the researchers from Aarhus and Copenhagen have published ground-breaking research results together in Nature. In 2007, the two research leaders Professor Poul Nissen and Professor Michael Broberg Palmgren published the world’s first structure of a biological pump from a plant. In close collaboration, they determined the structure of a proton pump from thale cress, and they explained how proton pumps can form the strong voltage across the plasma membrane that is a prerequisite for a plant’s uptake of nutrients from the soil.
Basic research is important for applied research
The main objective of the research into the regulation of biological pump activity is to find out how the control mechanisms work and which factors are involved in the regulation. These results may form the foundation for new breakthroughs in applied research.
Calcium pumps and the sodium-potassium pump are vital for the regulation of cardiac muscle activity, and are thus targets for already known drugs and the development of new ones for better treatment of cardiovascular diseases. The blocking of ion pump activity is also an obvious target for developing new drugs against cancer and infectious diseases.
Calmodulin is a calcium-binding protein – a type of calcium sensor. It is of great importance for the regulation of cellular activity in response to calcium, which is supplied to the cell at the opening of the ion channel receptors. The calcium-calmodulin complex activates a series of enzymes within the cell, such as protein kinases, which further regulate numerous other enzymes and factors. Calmodulin thus mediates signalling cascades within the cell – ‘it’s all about calcium’, as is often quoted.
Calcium pumps are made up of protein molecules that are also known as Ca2+-ATPases. Calcium pumps restore the calcium balance in human cells following calcium-mediated signalling, such as muscular effort, sensory input and nerve activity. The calcium pump belongs to a larger family of ion pumps called P-type ATPases, which are vital for life. The most well known of these is the sodium-potassium pump, which was discovered by Professor Jens Christian Skou, Aarhus University, and he was awarded the Nobel Prize in Chemistry for this in 1997.
Link to the research article in Nature:
A bimodular mechanism of calcium control in eukaryotes
Henning Tidow1,2*§, Lisbeth R. Poulsen1,3§, Antonina Andreeva4, Michael Knudsen1,5,
Kim L. Hein1,2#, Carsten Wiuf6, Michael G. Palmgren1,3 and Poul Nissen1,2*
1Centre for Membrane Pumps in Cells and Disease - PUMPKIN,
2Department of Molecular Biology and Genetics, Aarhus University, Gustav Wieds Vej 10c, 8000 Aarhus C, Denmark
3Department of Plant Biology and Biotechnology, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark
4MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 0QH, UK
5Bioinformatics Research Centre, Aarhus University, C.F. Møllers Allé 8, 8000 Aarhus C,
Denmark
6Department of Mathematical Sciences, University of Copenhagen, Universitetsparken 5, DK - 2100
Copenhagen, Denmark
*Corresponding authors: Henning Tidow, het@mb.au.dk and Poul Nissen, pn@mb.au.dk
#present address: Centre for Molecular Medicine Norway, Nordic EMBL Partnership, University of
Oslo, P.O. Box 1125, Blindern, N-0318 Oslo, Norway
§ H.T. and L.R.P. contributed equally
Contact
Postdoctoral Fellow Henning Tidow, +45 8942 5262, het@mb.au.dk
Professor Poul Nissen (Director of PUMPkin), +45 2899 2295, pn@mb.au.dk
Department of Molecular Biology and Genetics, PUMPkin
Aarhus University, Denmark
Postdoctoral Fellow Lisbeth Rosager Poulsen,, +45 3533 2595, lrpo@life.ku.dk
Professor Michael Broberg Palmgren, +45 3533 2592, palmgren@life.ku.dk
Department of Plant and Environmental Sciences, PUMPkin
University of Copenhagen, Denmark
Text: Inga Christensen Bach, Michael G. Palmgren (both the University of Copenhagen), Poul Nissen og Lisbeth Heilesen (both Aarhus University).
Translation into English: Lisbeth Heilesen
21 October 2012
