Electron microscopy allows scientists to understand the molecular trigger of allergic reactions
An international research team has been able to describe the overall structure of the antibody type IgE, which is the key molecule in allergic diseases. This is a scientific breakthrough which provides important insights into basic mechanisms of allergic reactions and may pave the way for more effective allergy medicine. The new research results have now been published in the scientific journal <em>Allergy</em>.
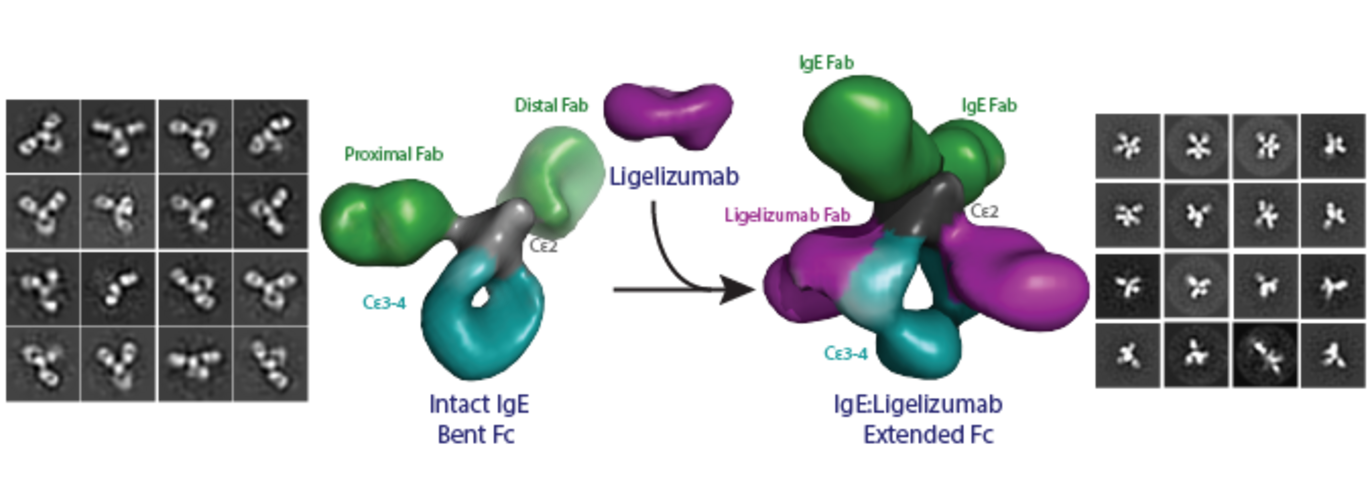
Antibodies are fundamental and versatile molecules of the human immune system. Different types of antibodies existing in humans share general features that include binding to potentially harmful antigens and the ability of inducing proper responses of the immune system. One of the tenets of immunobiology is that antibodies are flexible in order to maximise their efficacy.
The team of researchers from Aarhus, Denmark, and Marburg, Germany, used EM microscopy and small angle x-ray scattering for their analysis. At Aarhus University, Scientist Michaela Miehe and Postdoc Rasmus K Jensen worked closely together the past two years to achieve the results. Based on this work, the research team can now describe the three-dimensional structure of IgE. The results they obtained were very surprising to the scientists involved.
“For the first time, we could show that IgE antibodies are unique and are violating the dogma of antibody flexibility. My work with electron microscopy demonstrated directly that IgE is a highly rigid molecule with a defined architecture of the allergen-binding moieties, which is different from the behavior of the other antibody isotypes we know,” explains Postdoc Rasmus K Jensen.
The researchers also analyzed the structural and functional impact of a therapeutic antibody currently tested in clinical trials on the IgE molecule. This therapeutic antibody, which is of a different type than IgE, binds to IgE and prevents allergic reactions.
“Our new results describe the structural changes that IgE undergoes when neutralized by this anti-IgE antibody. This also allows us to understand better, how IgE recognizes allergens and the two IgE receptors sitting on the surface of the immune cells we have in our body,” explains Associate Professor Edzard Spillner.
Antibody features revised
Generally, an allergic person produces high levels of IgE molecules directed against external allergens when exposed to them. These IgE antibodies circulate in the blood and are loaded onto the effector cells of the immune system. Upon exposure to allergens, these armed cells harnessed with IgE are triggered to release large amounts of mediators and histamine and thereby cause an immediate allergic reaction in the body.
“We now realise that the picture we and other researchers have puzzled together through decades by studying IgE fragments differs compared to the new results obtained with intact IgE. It was also most satisfying that we could visualize how the anti-IgE antibody changes the IgE architecture including the antigen binding arms,” explains Professor Gregers Rom Andersen.
The researchers conducted their experiments with different recombinant IgE molecules they produced in the lab. These IgE molecules recognize specifically a house dust mite allergen and sugar groups found on allergens. However, the method can be transferred to virtually all types of IgE molecules.
Hope for better medicine
Allergic diseases are affecting the lives of more than one billion people worldwide, and their prevalence is expected to reach up to 4 billion in 2050. The prevalence of allergic diseases and socioeconomic impact are particularly on the rise in urbanizing regions and globalizing world in association with environmental and lifestyle changes. Apart from individual suffering of patients, allergic diseases present a very high cost for the health care systems. The current treatments cannot control all types of allergy, but the researchers now hope that their scientific results will pave the way for the development of new types of allergy medicine.
“We now understand much better and in much more detail the IgE molecule that we want to control and the way it behaves upon treatment of patients with allergy medicine. This also allows us to envision new strategies for developing medicine of the future,“ says Edzard Spillner.
While the obtained knowledge will finally benefit the individual patient, the team will also broaden the ongoing research in the field.
“The extraordinary characteristics of IgE makes it an exciting object for more detailed studies in concert with molecules within and outside the allergic immune response,” says Gregers Rom Andersen.
Overall, the team´s findings provide important basic and translational knowledge and are a starting point for further research, and perhaps for this reason, the research was also supported by the Novo Nordisk Foundation.
Contact
Associate Professor Edzard Spillner
The Department of Engineering, Aarhus University
e.spillner@eng.au.dk
Tel.: +45 21186396
Professor Gregers Rom Andersen
The Department of Molecular Biology and Genetics, Aarhus University
gra@mbg.au.dk
Tel.: +45 30256646
