Researchers reveal action mechanism of known food contaminant
The toxin cyclopiazonic acid (CPA) has been known for many years, but scientists have only just discovered how this mycotoxin works. This discovery will make it easier for researchers to develop new antibiotics.
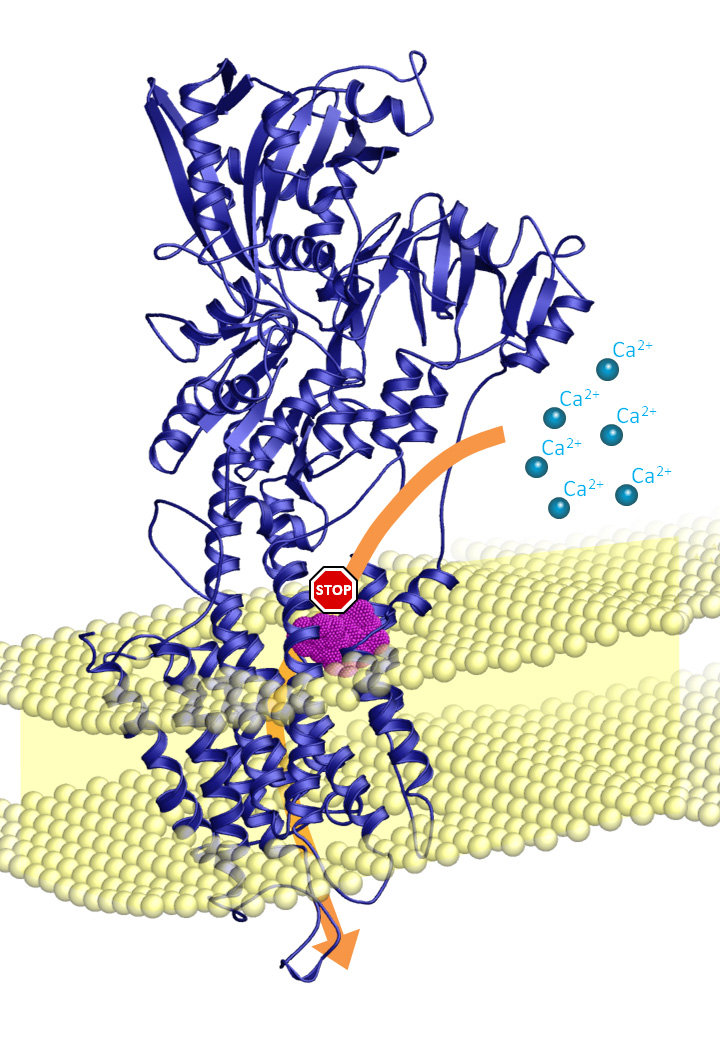
The toxin cyclopiazonic acid (CPA) was first isolated from a fungus (Penicillium cyclopium) back in 1968. The toxin is produced naturally by moulds which can infect food such as cereal and milk. It is produced in harmless quantities in mould cheese such as Camembert and Brie (Penicillium camemberti). Since the amounts produced are very small it represents no danger. However we know that if the cheese is stored at higher temperatures the fungi produce larger quantities of the toxin CPA.
Toxin attacks protein in the body
Ingestion of small quantities of the toxin CPA is not harmful, but larger quantities of CPA may have a negative effect since the CPA attacks a protein in the body called the calcium pump. This pump is vital for the function of muscles and for the maintenance of the right level of calcium in all the cells of the body. The toxin CPA slows down this pump, which among other things causes diarrhea and nausea, and in large doses it even causes life-threatening symptoms.
Identification of the effect of the toxin
After several years of study researchers have now obtained results providing a detailed insight into how the CPA works. With this knowledge the researchers will be able to identify and develop variants of the CPA which can act specifically on, for example, the calcium pumps of the tuberculosis bacteria and malaria parasites. This knowledge could form the basis for the development of new drugs/antibiotics to control of the above disease-causing microorganisms.
These important results have just been published online in the internationally recognized journal, Journal of Biological Chemistry.
The research group behind the identification includes among others Graduate Student Mette Laursen and Postdoc Maike Bublitz headed by Assistant Professor J. Preben Morth from the Department of Molecular Biology, Aarhus University, Denmark. The discovery was made in collaboration with a Canadian group.
Link to the published article:
Mette Laursen, Maike Bublitz, Karine Moncoq, Claus Olesen, Jesper Vuust Møller, Howard S. Young, Poul Nissen and Jens Preben Morth: Cyclopiazonic acid is complexed to a divalent metal ion when bound to the sarcoplasmic reticulum Ca2+-ATPase.
The group from Aarhus has been studying the molecular pump for more than 30 years.
More information
For further information please contact Assistant Professor J. Preben Morth, Department of Molecular Biology, The PUMPIN Centre, Aarhus University. Tel.: +45 8942 5257, Mobile: +45 6170 5045.
