Llama-derived nanobodies as a new tool in solving crystal structure
Aarhus University scientists have developed miniature antibodies (nanobodies) that can be labelled on certain amino acids. This provides a direct route for solving new X-ray crystal structures of protein complexes important for gaining mechanistic understanding of cellular processes, which is important in the development of drugs.
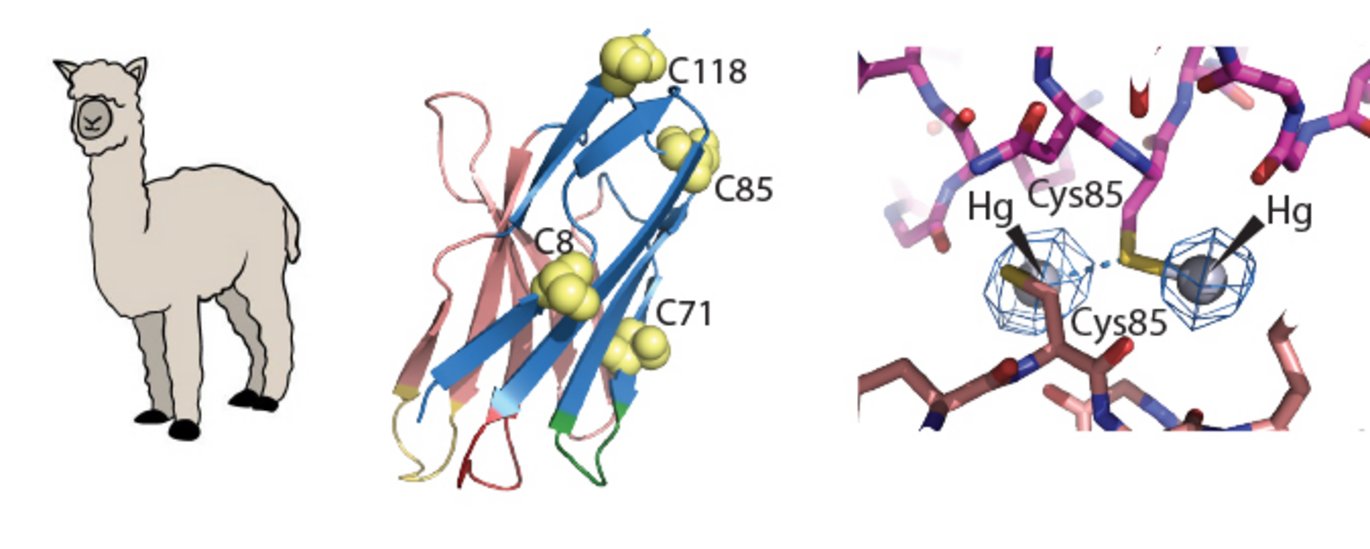
Nanobodies are miniature antibodies derived from naturally circulating heavy-chain only antibodies in llamas. Over the past years, nanobodies and their applications have expanded enormously, both in basic research but also in drug development.
Nanobodies have proven to be well suited as protein stabilizers, which is particularly important during crystallization of a protein where millions of molecules have to arrange in a well-defined lattice. In this way, nanobodies can act as crystallization chaperones.
In an X-ray diffraction experiment, a critical piece of information - called the phases - is lost, which makes it difficult to determine new crystal structures. To overcome this phase problem in crystallography, heavy atoms are needed in the crystal. However, it is challenging to insert heavy atoms into a crystal. The scientists at Aarhus University used a nanobody as the vehicle for introducing mercury atoms. They developed a method to site-specifically label the nanobody with a heavy atom, and in this way, they could overcome the phase problem.
Since the scientists know which specific residues in the nanobodies can be modified and labelled, the technique used at Aarhus University opens for a range of other application. One exciting perspective is the insertion of fluorescent dyes into the nanobody to follow the location and distribution of target proteins in living organisms, which can give essential information on functional and regulatory processes.
Link to the scientific article published in Acta Crystallographica Section D. “Introducing site-specific cysteines into nanobodies for mercury labelling allows de novo phasing of their crystal structures”.
More information
Assistant Professor Kasper Røjkjær Andersen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
kra@mbg.au.dk
Assistant Professor Nick Stub Laursen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
nls@mbg.au.dk
