Molecular Velcro helps to assemble functional nuclear pore complexes
An international research team now explains how one of the largest molecular machineries - the nuclear pore complex – is being assembled using natively unfolded FG-repeats as molecular Velcro.
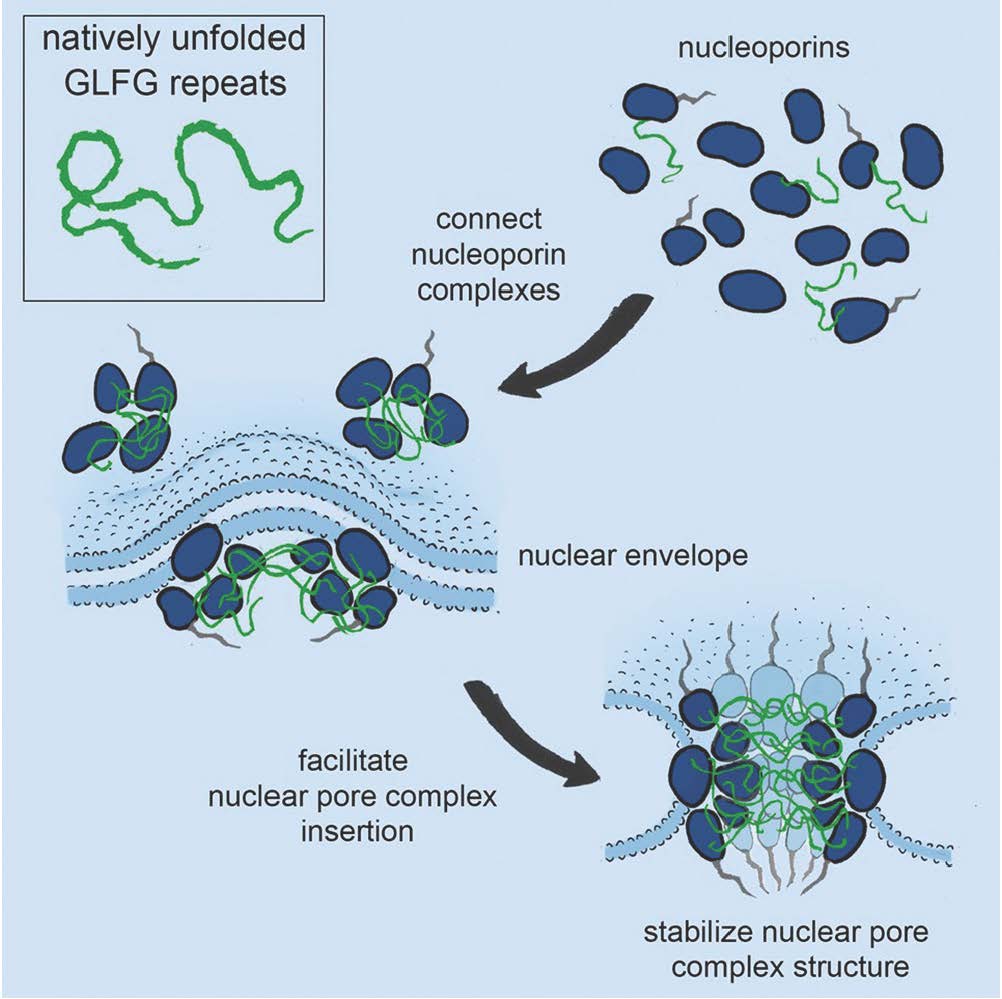
The nuclear pore complex is one of the largest and most complex structures in our cells. This pore constitutes the only passage between the nucleus and cytoplasm and is thus responsible for all transport of macromolecules (such as RNA and proteins) into and out of the cell nucleus. The nuclear pore complex is composed of about 500 individual proteins (nucleoporins), which assemble into functional pores. Some nucleoporins are structural scaffold proteins and other form a central hygrogel that accommodates selective transport. This hydrogel forms the diffusion barrier and is built of natively unfolded phenylalanine-glycine (FG)-rich repeats, which is selectively permeable for nuclear transport receptors that carry other macromolecules across.
The research team from USA, Switzerland and Denmark recently discovered that some nucleoporins are structurally similar to nuclear transport receptors and that they specifically bind FG-repeats, but the functional role of these interaction has until now been a mystery. In a new paper published in Cell, the researchers show that FG-repeats work as molecular Velcro important for the structure and de novo assembling of nuclear pore complexes, which brings us close to understanding this giant molecular machine.
Thomas Schwartz from MIT, who is one of the senior authors, is visiting MBG and will give a Kjeldgaard Lecture, entitled ”The machinery that enables nuclear communication” on Thursday 7 December 2017.
More information
Assistant Professor Kasper Røjkjær Andersen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
kra@mbg.au.dk
