New connection between early transcription termination and RNA turnover
Transcription is the process through which DNA is copied to RNA. Surprisingly, only a minority of transcription events reach completion, supposedly due to quality control of the transcription reaction. New results now add to the pathways by which this is achieved.
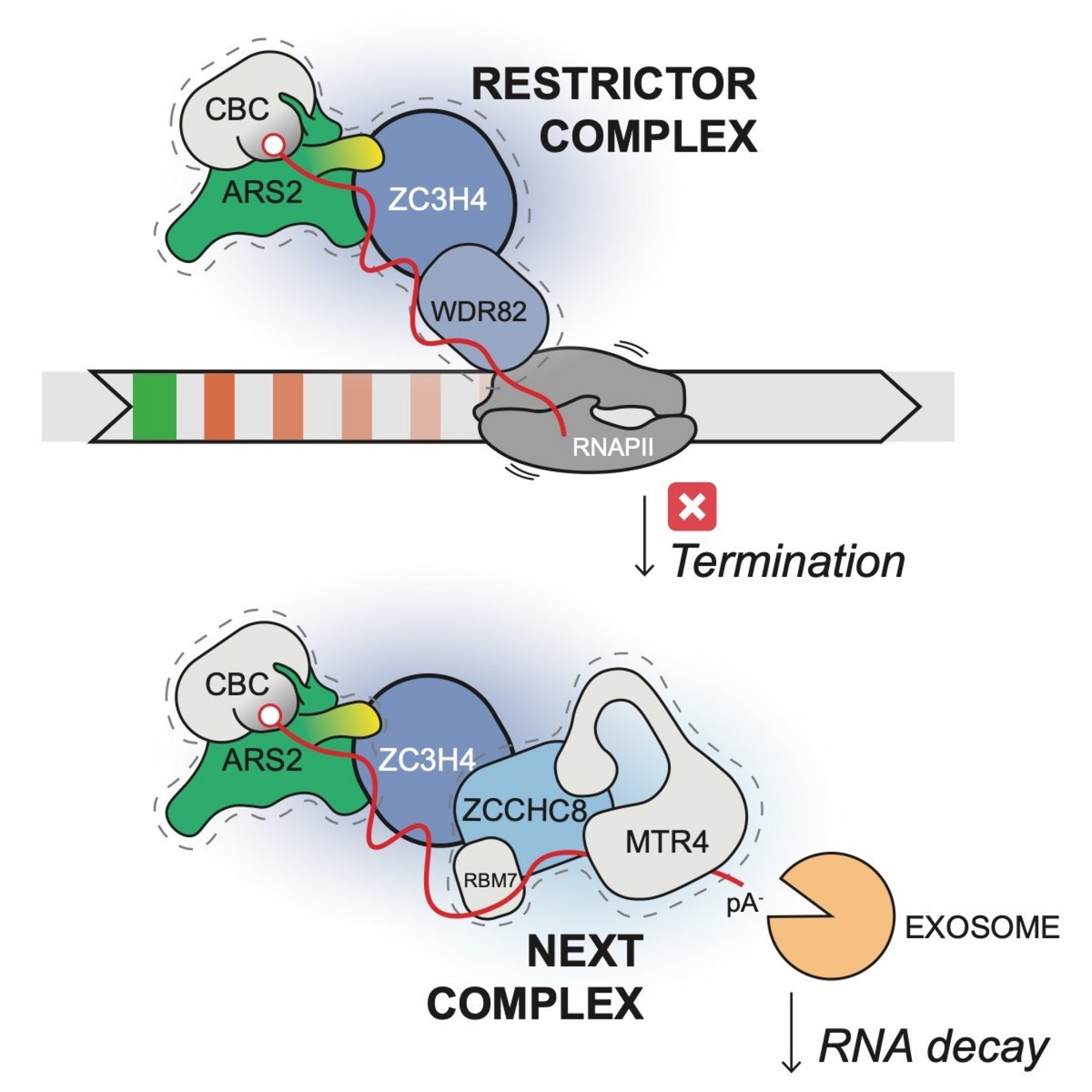
An international collaboration between two Danish and two German teams has delineated that the so-called ‘Arsenite resistance 2 (ARS2)’ protein is capable of inducing early transcription termination by recruiting the termination factors ZC3H4/WDR82 via a conserved protein-protein interaction. In turn, it was found that ZC3H4 associates with the ‘nuclear exosome targeting (NEXT)’ complex, providing access to the nuclear RNA degradation machinery. This reveals, for the first time, in higher eukaryotes, a direct coupling between termination of a transcription reaction and the degradation of its cognate RNA product (see figure).
Previous to the present work, the Heick Jensen laboratory had contributed to the description of ARS2 as an interaction 'hub' for a wide range of proteins involved in RNA maturation and turnover. Depending on its associated factor(s), ARS2 was suggested to impact RNA 'fate', determining its degradation or its export out of the nucleus. Conspicuously, however, depletion of ARS2 also displayed a transcription termination phenotype, the nature of which had remained unexplained.
So to investigate possible action mechanisms, postdoc Jerome Rouviere, set out to purify ARS2 from cells and characterize its associated protein partners by mass spectrometry (MS). Notably, the transcription restriction factor ZC3H4 was identified. Upon further investigation, Jerome could show that ZC3H4 interacts with ARS2 by means of a highly conserved ‘short linear motif (SLiM)’, and in doing so, ZC3H4 gets recruited to chromatin.
Additional MS analysis of purified ZC3H4 complexes highlighted, that the protein also interacts with the ZCCHC8 component of the NEXT complex. This dual effect of ZC3H4 in transcription termination and RNA decay presumably ensures that the useless product of the premature termination event is rapidly removed to prevent contamination of cells with excess RNA.
The findings derive from a collaborative project between the laboratories of Torben Heick Jensen, Department of Molecular Biology and Genetics, Aarhus University, Elena Conti, Department of Structural Cell Biology, Max Planck Institute of Biochemistry, Martinsried, Jens S. Andersen, Department of Biochemistry and Molecular Biology, University of Southern Denmark and Patrick Cramer, Department of Molecular Biology, Max Planck Institute for Multidisciplinary Sciences, Goettingen.
The study was published in the internationally recognized journal Molecular Cell.
For further information, please contact
Professor Torben Heick Jensen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
thj@mbg.au.dk – mobil: +45 60202705
SUPPLEMENTARY INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
Study type | Experiment |
External funding | T.H.J. laboratory was supported by the Danish National Research Council, the Lundbeck Foundation (R1982015-172), and the Novo Nordisk Foundation (NNF18OC0033380), and (ExoAdapt grant 31199). J.O.R. is supported by a Lundbeck Foundation Postdoc grant (R303-2018-3134). Work in the E.C. laboratory was supported by the Max Planck Society, the Novo Nordisk Foundation (ExoAdapt grant 31199), the European Research Council (EXORICO ERC Advanced Grant 740329), and the German Research Foundation (DFG SFB 1035, GRK 1721, SFB/TRR 237). Work in the J.S.A. laboratory was supported by the Novo Nordisk Foundation (ExoAdapt grant 31199). Work in the P.C. laboratory was supported by the Deutsche Forschungsgemeinschaft (SFB860, SPP1935, EXC 2067/1-390729940) and the European Research Council (advanced investigator grant CHROMATRANS, grant agreement no. 882357). |
Conflict of interest | None |
Link to the scientific paper | Jérôme O. Rovière, Anna Salerno-Kochan, Søren Lykke-Andersen, William Garland, Yuhui Dou, Om Rathore, Ewa Smidová Molska, Guifen Wu, Manfred Schmid, Andrii Bugai, Lis Jakobsen, Kristina Zumer, Patrick Cramer, Jens S. Andersen, Elena Conti, and Torben Heick Jensen ARS2 instructs early transcription termination-coupled RNA decay by recruiting ZC3H4 to nascent transcripts Molecular Cell |
