Protein pumps copper, silver and gold
Researchers from Aarhus University are the first in the world to map the structure of a protein that pumps copper ions across the cell membrane. Almost all organisms are dependent on this protein to control the cell levels of copper, which is essential in small quantities but deadly toxic at elevated concentrations. The findings have been published in the leading scientific journal Nature.
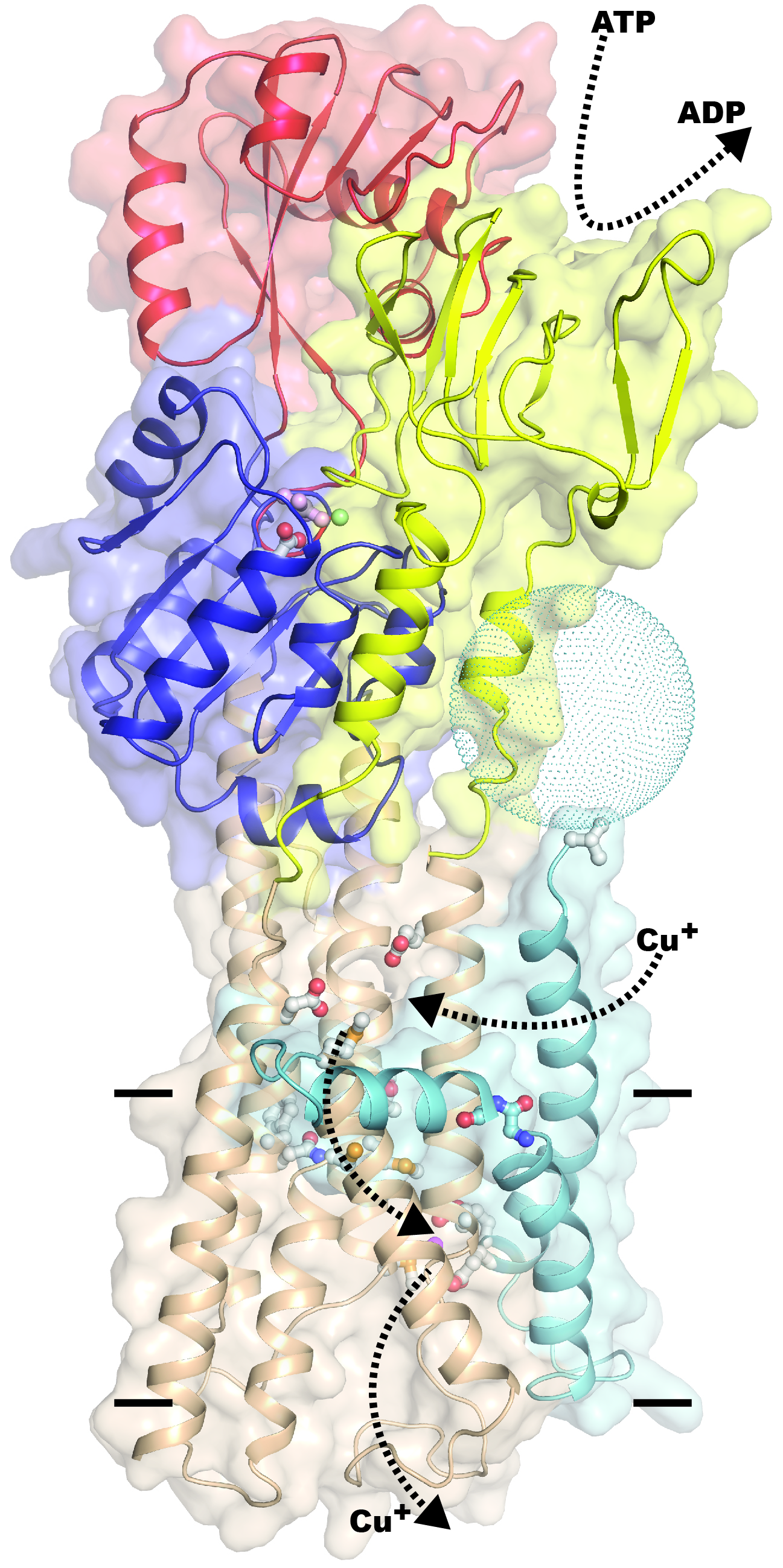
The protein is a so-called ATP-driven ion pump, known as Cu+-ATPase or CopA. In addition to copper ions, CopA reactsto silver and gold ions. The general mechanism of the protein is to bind metal ions from one side of a cell membrane using energy liberated by ATP splicing (a so-called ATPase activity) to pump them to the other side. This can also occur from low to high concentration – i.e. as a concentration of metal ions.
The protein’s essential function in the cell and its unique properties in interesting metals offer many new and exciting applications – both as targets for new drugs against cancer, parasites and pathogenic bacteria, for example, and as a new biotechnological tool to extract valuable metals like copper, silver and gold.
The CopA protein the researchers studied specifically is from the bacterium Legionella pneumophila, which causes the deadly legionnaires’ disease (but here cloned into a harmless coli bacterial strain). Since the Legionella bacterium is dependent on a functional copper pump that could infect humans, the CopA protein – which is almost identical in all organisms – is an exciting target for new antibiotics that can also be effective against a wide range of other difficult infections such as multidrug-resistant bacteria and TB.
An international research team at the Danish National Research Centre Pumpkin, Department of Molecular Biology and Genetics, Aarhus University, was responsible for the major part of the research project. Led by Professor and Centre Director Poul Nissen, the team consisted of Postdoctoral Fellow Pontus Gourdon (Swedish Research Council), visiting student Xiang-Yu Liu (Peking University) and Carlsberg Fellow Bjørn P. Pedersen (now at the University of California, San Francisco).
The Pumpkin research team also collaborated with geneticists from the Kennedy Center at Glostrup led by Lisbeth Birk Møller to uncover new knowledge about genetic diseases related to mutations in the human copper pumps.
See movie with the structure of the ion pump
(after starting, click the arrow in front of Vimeo for larger version)
Long, detailed version (2:19 min)
Short version (1:24 min)
Link to the article:
Crystal structure of a copper-transporting PIB-type ATPase
Pontus Gourdon1,2*, Xiang-Yu Liu1,2,3*, Tina Skjørringe4, J. Preben Morth1,2#, Lisbeth Birk Møller4, Bjørn Panyella Pedersen1,2† & Poul Nissen1,2
1Centre for Membrane Pumps in Cells and Disease – PUMPKIN, Danish National Research Foundation.
2 Department of Molecular Biology, Aarhus University, Gustav Wieds Vej 10C, DK-8000 Aarhus C, Denmark.
3State Key Laboratory of Protein and Plant Gene Research, College of Life Sciences, Peking University, Beijing, 100871, P.R. China.
4Center for Applied Human Molecular Genetics, Kennedy Center, Gl. Landevej 7, 2600,Glostrup, Denmark.
† Present address: Department of Biochemistry and Biophysics, University of California at San Francisco, San Francisco, California 94158, USA.
# Present address: The Biotechnology Centre of Oslo and Centre for Molecular Medicine, Nordic EMBL Partnership, University of Oslo, Oslo, Norway.
* These authors contributed equally to this work.
More information
Professor Poul Nissen
Department of Molecular Biology and Genetics, Aarhus University, Denmark
pn@mb.au.dk, + 45 2899 2295 (after noon due to stay in the USA)
Postdoctoral Fellow Pontus Gourdon
Department of Molecular Biology and Genetics, Aarhus University, Denmark
pgo@mb.au.dk, + 45 5033 9990
Text: Poul Nissen, Pontus Gourdon and Lisbeth Heilesen
Translation: Lisbeth Heilesen
