Researchers discover how bacteria sweet-talk their way into plants
An international team of researchers has discovered how legumes are able to tell helpful and harmful invading bacteria apart. The research has implications for improving the understanding of how other plants, animals and humans interact with bacteria in their environment and defend themselves against hostile infections. These findings can have profound implications for both agricultural research and medical science.
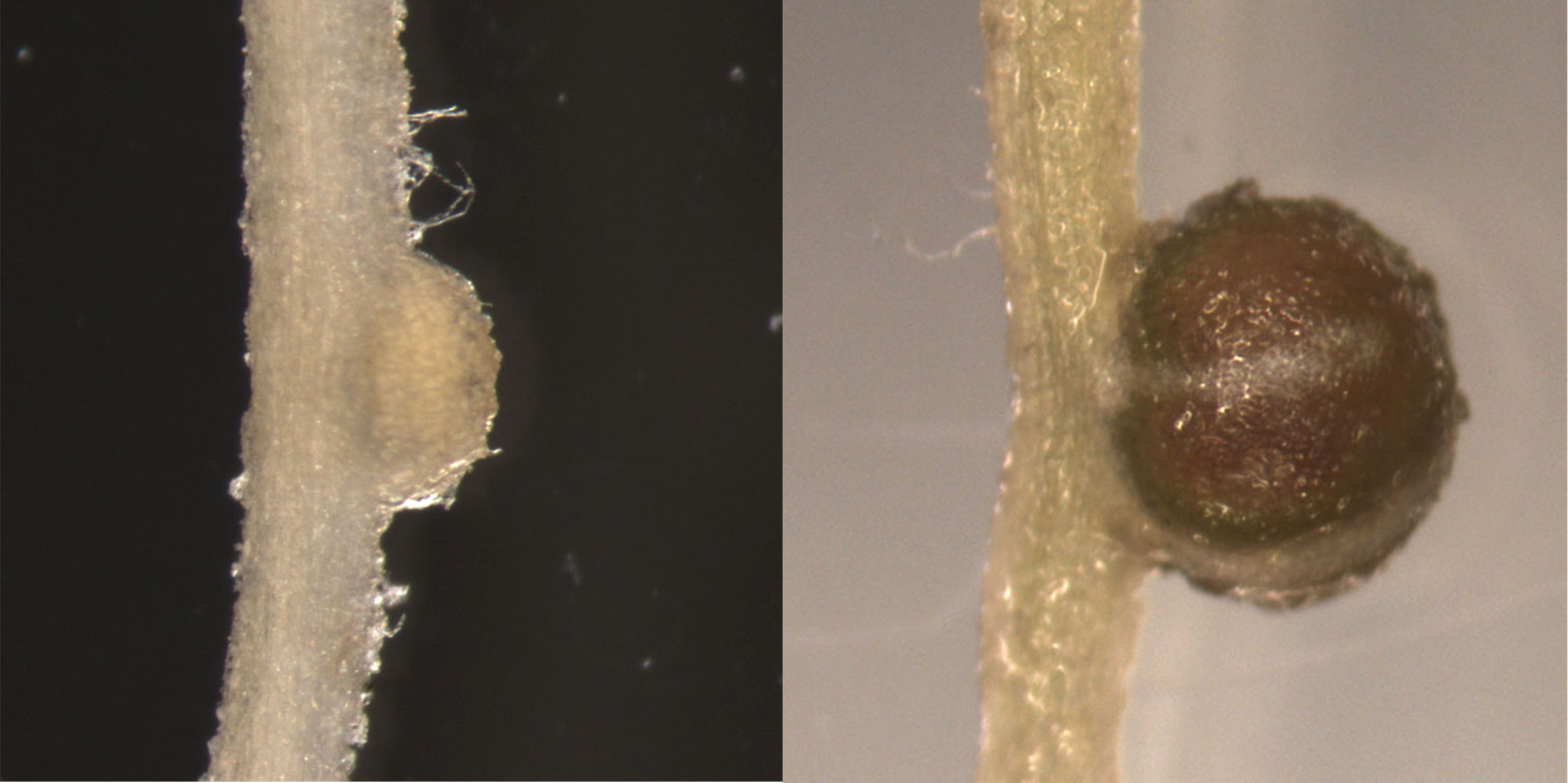
Their study, which changes the understanding of carbohydrates as signal molecules, is newly published in the leading international journal Nature.
Legumes form a unique symbiotic relationship with bacteria known as rhizobia, which they allow to infect their roots. This leads to root nodules being formed in which the bacteria convert nitrogen from the air into ammonia that the plant can use for growth.
Exactly how these plants are able to distinguish and welcome compatible rhizobia for this self-fertilising activity – while halting infection by incompatible bacteria – has been a mystery.
Now the researchers at the Centre for Carbohydrate Recognition and Signalling (CARB) from Denmark and New Zealand and their collaborators from the Complex Carbohydrate Research Center, the University of Georgia, Athens, Georgia, USA, have determined how legumes perceive and distinguish compatible bacteria based on the exopolysaccharides featuring on the invading cells’ surfaces.
Using an interdisciplinary approach involving plant and microbial genetics, biochemistry and carbohydrate chemistry, the researchers have identified the first known exopolysaccharide receptor gene, called Epr3.
They found that a membrane-bound receptor kinase encoded by the Epr3 gene binds directly with exopolysaccharides and regulates beneficial bacteria’s passage through the plant’s epidermal cell layer.
The Director of CARB, Professor Jens Stougaard, says that “this exceptional achievement is a result of a unique interdisciplinary collaboration possible within centres of excellence”.
Bacteria display a wide selection of polysaccharides, and many of these are important for cell-to-cell interactions, immune evasion, pathogenesis, biofilm formation and colonisation of ecological niches.
“Now that we have identified this key mechanism that allows a host organism to distinguish friendly bacteria from those that cause disease, this opens up exciting new research avenues across a number of fields,” says Professor Stougaard.
Microbiome studies in plants, animals and humans are some of the areas that will benefit from the new discovery. The mechanism governing microbiota colonisation of hosts is poorly understood and the identification of an exopolysaccharide receptor is likely to inspire new approaches to understand the interaction between multicellular organisms and microbes.
Link to the scientific article in Nature: Receptor-mediated exopolysaccharide perception controls bacterial infection.
Nature: News and Views: Symbiosis: Receptive to infection.
CARB is a centre of excellence funded by the Danish National Research Foundation – with researchers from Denmark (Aarhus University and the University of Copenhagen) and New Zealand (University of Otago). In addition the collaboration involved researchers from the USA (the Complex Carbohydrate Research Center, the University of Georgia, Athens, Georgia).
For more information, contact:
Professor Jens Stougaard
Department of Molecular Biology and Genetics
Aarhus University, Denmark
stougaard@mbg.au.dk - Cell: 004560202649
