Structure of the lipid flippase ATP8B1 reveals important mechanisms of regulation
The human flippase ATP8B1 is critical for proper excretion of bile in the liver, and mutations of ATP8B1 are associated with severe liver diseases. The three-dimensional structure by cryo-EM along with functional studies reveal how ATP8B1 is autoinhibited through its N- and C-terminal segments and contribute to the understanding of regulatory mechanisms of ATP8B1 that may also open up new opportunities for drug development.
Progressive familial intrahepatic cholestasis (PFIC) is a rare, inherited liver disease resulting of impaired bile production or flow. This causes liver damage and in severe forms, PFIC may progress toward liver failure and death before adulthood. The type 1 PFIC is caused by mutations of the human ATP8B1. This protein is a member of the P4-ATPase family, also known as lipid flippases, which transport phospholipid across cellular membrane. ATP8B1’s function as a lipid flippase contributes to the regulation of lipid distribution of the membrane across the canalicular membrane of hepatocytes to withstand the excretion of bile acids. ATP8B1 is also widely expressed in many other tissues such as the heart and the brain, and a genome-wide association study have indicated that regulation of ATP8B1 expression is strong factor in resilience to Alzheimer’s disease.
A collaboration between researchers from the University of Paris-Saclay, Ruhr University Bochum and Aarhus University has explored the structure, function and regulation of the ATP8B1-CDC50A complex. Full-length ATP8B1 was found to be inactive. However, truncation of the C-terminal region of ATP8B1 resulted in activation of the ATPase activity of the complex. This activity was further increased when N-terminal region of ATP8B1 was also truncated, revealing a cooperative autoinhibition mechanism. Activity assays of the terminally truncated ATP8B1 further revealed that phosphoinositide lipids are required as a co-factor to elicit the transport of phospholipids.
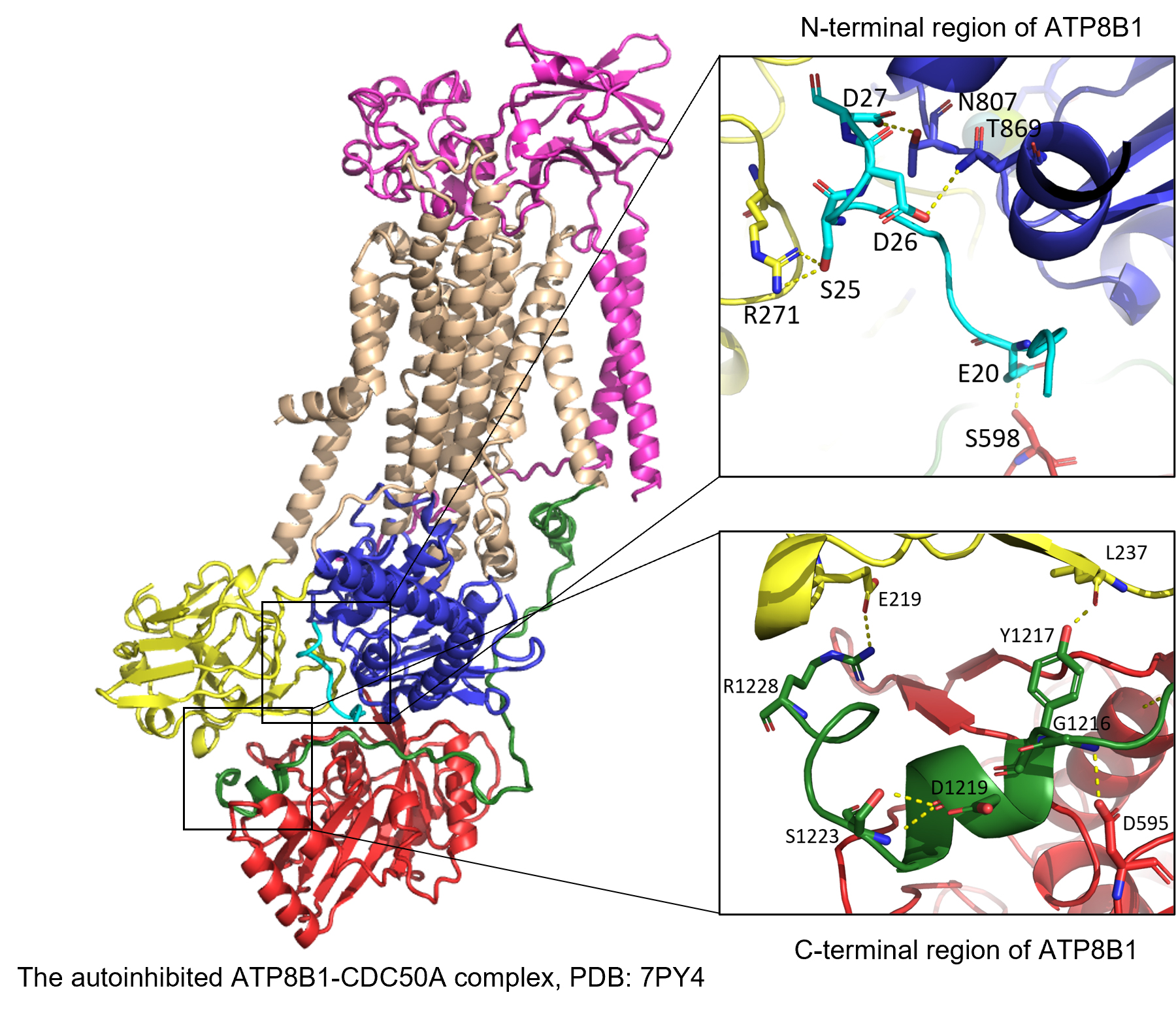
Using Cryo-EM, the structure of the autoinhibited ATP8B1 in complex with CDC50A was determined to an overall resolution of 3.1Å. ATP8B1 has a P-type ATPase topology, with a transmembrane domain (shown in wheat) and three intracellular domains known as the P-, N- and A-domain shown in blue, red, and yellow respectively. Importantly, the structure revealed that both the N- and C-terminal regions bind to all three intracellular domains in a manner that prevents conformational transition associated with the transport cycle. Furthermore, a residue located in the C-terminal region, Ser-1223, is engaged in intimate interactions and has been found to be phosphorylated in a phosphoproteomic study in mouse. Ser-1223 is conserved in human ATP8B1.
Because of the intimate interactions of Ser-1223, phosphorylation at this site would be expected to affect the binding properties and inhibition of the C-terminal region. To further explore the effect of the phosphorylation, the truncated ATP8B1 was treated with peptides equivalent to the missing C-terminal region with and without a phosphorylation at Ser-1223. Indeed, phosphorylation decreased the inhibition 25-fold, indicating that phosphorylation of Ser-1223 may be part of the mechanism that promotes the release of the autoinhibitory C-terminus and thus the activation of ATP8B1.
Our new knowledge on regulatory mechanisms of ATP8B1 suggests that molecules that either mimic the effect of autoinhibition or lift the autoinhibition by the C-terminal region could regulate ATP8B1 activity and may open for new drug intervention for e.g. liver disorders and alzheimer’s disease.
SUPPLEMENTARY INFORMATION, INCLUDING CONTACT INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
Study type | Experiment |
External funding | EMBO (Short-term fellowship 7881): Thibaud Dieudonné French Infrastructure for Integrated Structural Biology (FRISBI ANR-10-INSB-05): Christine Jaxel, Cédric Montigny, Guillaume Lenoir French Ministry for Higher Education (PhD fellowship): Thibaud Dieudonné European Commission (Marie Sklodowska-Curie individual fellowship): Thibaud Dieudonné Agence Nationale de la Recherche (Young Investigator grant ANR-14-CE09-0022): Guillaume Lenoir Lundbeckfonden (Professorship grant): Poul Nissen Deutsche Forschungsgemeinschaft (GU 1133/11-1): Thomas Günter Pomorski Dansih Agency for Science and Higher Education (5072-00025B – Danish National Cryo-EM Research Infrastructure (EMBION)): Poul Nissen The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. |
Conflicts of interest | Forskerne erklærer at der ingen interessekonflikt er. |
Link to scientific paper | Autoinhibition and regulation by phosphoinositides of ATP8B1, a human lipid flippase associated with intrahepatic cholestatic disorders Thibaud Dieudonné, Sara Abad Herrera, Michelle Juknaviciute Laursen, Maylis Lejeun, Charlott Stock, Kahina Slimani, Christine Jaxel, Joseph A Lyons, Cédric Montigny, Thomas Günther Pomorski, Poul Nissen, Gillaume Lenoir eLife 11:e75727 |
Contact information | Professor Poul Nissen |
