A structural explanation for the unique mechanism of protease inhibition by the A2M family of proteins
A group of scientists from the Department of Molecular Biology and Genetics, Aarhus University, has determined the structures of a protease inhibitor called A2ML1 that is found in human skin. The new results reveal how a large conformational rearrangement in A2ML1 allows it to trap a protease and prevents it from cleaving substrates.
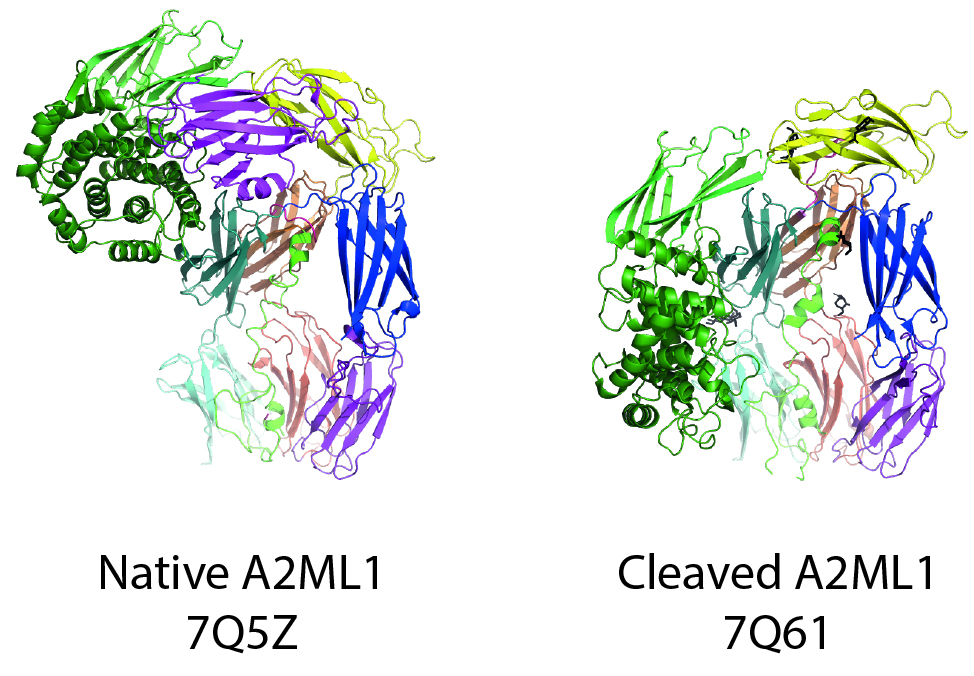
Alpha macroglobulin (AM) protease inhibitors utilize a unique trapping mechanism that allows them to inhibit proteases of any catalytic class. The best-known AM protease inhibitor is A2M, a large tetramer that circulates in the blood at high concentrations.
Both professors involved in this study, Jan J. Enghild and Gregers R. Andersen, began studying the structure and function of A2M and related proteins over 30 years ago. However, the structural determination of A2M turned out to be complicated by its large size and an unusual non-compact structure. Hence, when Jan Enghild became aware of the monomeric AM protease inhibitor alpha-2 macroglobulin-like 1 (A2ML1) present in the skin of humans, he saw the chance to return to an unfinished chapter from his earlier work.
SeanDean Lykke Harwood was working as a PhD student in Jan Enghild’s lab, and he swiftly established a protocol for recombinant expression and purification of A2ML1. Postdoc Nadia Sukusu Nielsen began to carry out biochemical experiments to study the function of A2ML1 and soon, Gregers Rom Andersen was persuaded to aid with the structural determination of the protein.
Through hard work, Nadia prepared crystals of A2ML1 and a rough outline of the molecule could be seen based on these crystallographic data, but the atomic details remained a mystery.
In the meantime, a new cryo-EM facility at Aarhus University became operational. Postdoc Alessandra Zarantonello working with Gregers started preparing grids for cryo-EM to try out this new structure determination method. Using one of the first cryo-EM 3D maps obtained by Alessandra, the interpretation of the crystallography results improved enormously. Gregers was impressed and decided to have Alessandra focus entirely on cryo-EM.
The collaborative efforts of Nadia and Alessandra continued, as a single structure was not enough! They would only be satisfied once they had structures of A2ML1 in complex with a protease. To achieve this, Nadia prepared multiple high-quality samples where A2ML1 was very specifically cleaved by a protease and the protease-A2ML1 complexes were isolated through optimized purification protocols. These samples gave even better 3D cryo-EM maps than the initial uncleaved A2ML1 samples. Throughout the process, Alessandra and Nadia had excellent support from the staff at the cryo-EM facility at Aarhus University, which allowed them to collect data from home during the lockdowns imposed by the pandemic.
The research is now published in the prestigious scientific journal Nature Communications. The work leads to a detailed understanding of the function of A2ML1, but also improves the understanding of other related proteins in humans. From a human health perspective, A2ML1 has been linked to a severe autoimmune blistering disease with poor treatment options, and the new results clarify why the function of A2ML1 is disrupted by these disease-associated mutations.
SUPPLEMENTARY INFORMATION, INCLUDING CONTACT INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
Study type | Experiment |
External funding | The Independent Research Foundation Denmark, the LEO Foundation, the VELUX Foundation, the Novo Nordisk Foundation, and the Lundbeck Foundation |
Conflicts of interest | The researchers declare that there are no conflicts of interest. |
Link to scientific paper | Cryo-EM structures of human A2ML1 elucidate the protease-inhibitory mechanism of the A2M family Nadia Sukusu Nielsen, Alessandra Zarantonello, Seandean Lykke Harwood, Kathrine Tejlgård Jensen, Katarzyna Kjøge, Ida B. Thøgersen, Leif Schauser, Jesper Lykkegaard Karlsen, Gregers R. Andersen, Jan J. Enghild Nature Communications |
Contact information | Professor Jan J. Enghild Institut for Molekylærbiologi og Genetik Aarhus Universitet |
