Researchers reveal the structure of the IFT-B complex, which is essential for formation of the cilium organelle
Defects in the cell's cilia are the cause of many different diseases. Therefore, there is a great focus on identifying the function of cilia, so that drugs can be developed against these diseases. A research team has now taken another step towards this, as they have uncovered the structure of the IFT-B complex, which is crucial for the formation of cilium organelles.
Cilia are organelles that provide eukaryotic cells with motility, sensory reception and intracellular transduction of external stimuli. They are constructed and maintained by the bi-directional movement of 22 different proteins that organizes into IFT-A and IFT-B complexes powered by molecular motors.
Failure to construct cilia in human cells results in syndromic diseases known as ciliopathies that can manifest as deafness, blindness, obesity, inversed positioning of organs, accumulations of liquid in the brain, formation of cysts in the kidney, skeletal abnormalities, polydactyly, infertility and recurrent lung infections.
Although the first structural analysis of IFT proteins were published 11 ago, the complete structural analysis of larger IFT complexes has been hampered by technical difficulties and posed significant challenges. These include the high degree of flexibility and multiple conformations adopted by IFT complexes, which is likely necessary to orchestrate interactions with multiple different cargo proteins.
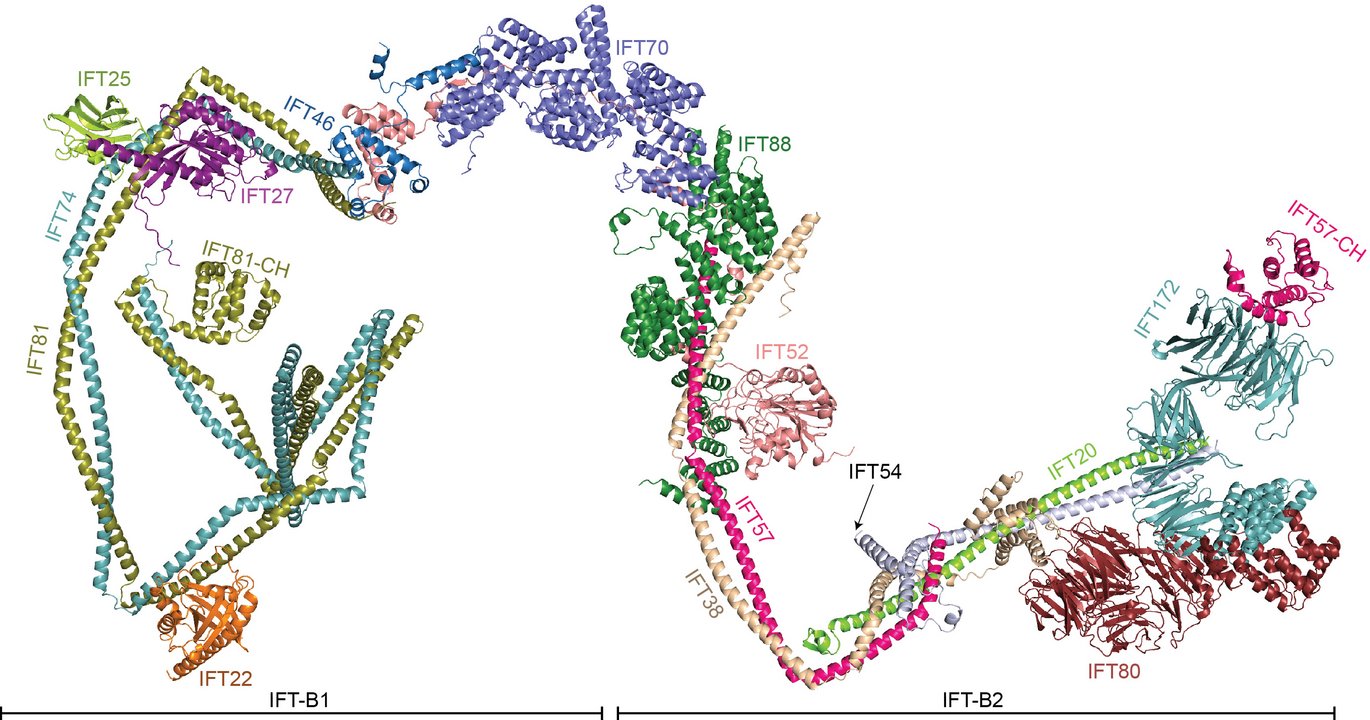
Now a team of researchers from the Laboratory of Esben Lorentzen at the Department of Molecular Biology and Genetics, Aarhus University, together with research teams from Odense, Germany and Switzerland, used recent advances in structure prediction to reveal the structure of the 15-subunit IFT-B complex. The results are published as a research article in the EMBO Journal.
With the new results, another step has been taken to gain more knowledge about cilia so that in the long term, new drugs can be developed that can fight the diseases associated with the lack of construction of cilia.
From structure prediction towards experimental validation and biological impact
The structural model of the almost complete IFT-B complex represents the culmination of more than a decade of research into the mechanisms of cilium formation.
The IFT-B structure reveals an elongated and highly flexible complex consistent with previous low-resolution in situ cryo-electron tomographic reconstructions of IFT proteins polymerized into trains. Interestingly, the new data reveal two configurations of the IFT-B complex that may reflect the conformational changes necessary to drive the IFT trains bi-directionally.
The research group’s data present a structural framework to understand IFT-B complex assembly, function, and ciliopathy variants. IFT is essential for cilium formation and organismic development as highlighted by IFT54 mutant mice that cannot form cilia and thus fail in proper embryonic development. Patients suffering from ciliopathies caused by mutations in IFT-B genes are thus expected to produce viable IFT particles that support at least some degree of cilium formation and function. The researchers mapped onto IFT-B structure over 15 prominent ciliopathy mutations causing Bardet-Biedl syndrome, short-rib thoracic dysplasia or asphyxiating thoracic dystrophy. These disease variants tend to interfere with IFT-B stability and cargo loading, particularly for the most severe ciliopathies.
SUPPLEMENTARY INFORMATION, INCLUDING CONTACT INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
Study type | Experiment |
External funding | This work has been supported by the Novo Nordisk Foundation (grant number NNF15OC00114164) and the Independent Research Fund Denmark (grant no: 1026-00016B) to E.L. N.A.P was supported by a postdoc fellowship from the European Commission (H2020, Grant Agreement number 888322). R.B.R. and E.L. have received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 861329. M.L.L and J.S.A was supported by the Independent Research Fund Denmark (grant no: 8021-00425B) to J.S.A. |
Conflicts of interest | The researchers declare that there are no conflicts of interest. |
Link to scientific paper | Narcis A. Petriman, Marta Loureiro-López, Michael Taschner, Nevin K. Zacharia, Magdalena M. Georgieva, Niels Boegholm, Jiaolong Wang, André Mourão, Robert B. Russell, Jens S. Andersen and Esben Lorentzen Biochemically validated structural model of the 15-subunit IFT-B complex EMBO Journal |
Contact information | Postdoc Narcis A. Petriman Associate Professor Esben Lorentzen |
