The dual role of well-known protein sheds light on Parkinson's disease mechanisms
For years, clumping of the alpha-synuclein protein (aSN) has been known as a central player in diseases that break down the brain and its functions. However, the function of native, soluble aSN has been unknown. But new research from Aarhus University with international collaborations has revealed that natural aSN activates an important calcium pump in the cell membrane. Regulation of calcium signals is thus disrupted by the aggregation of aSN. This discovery is an important step towards understanding the complex biological mechanism behind diseases such as Parkinson's in order to ultimately cure them.
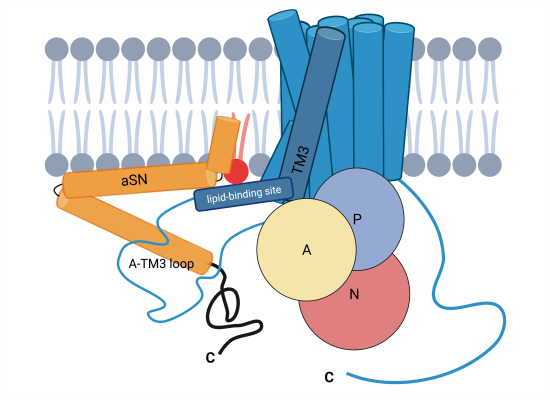
The protein alpha-synuclein (aSN) has long been known as a main cause in Parkinson's disease and Lewy Body Dementia, for example, when it forms lumpy protein aggregates that destroy cell function - but aSN in its natural form, without clumping, has not had a concrete, known function.
An international research team – led by Poul Nissen and Poul Henning Jensen from DANDRITE – has now demonstrated that natural aSN is an important activator of an essential calcium pump in the cell membrane.
Previously, the protein calmodulin was the only known activation factor of PMCA, but the researchers now add another important player and show that the activation of PMCA by aSN takes place in negatively charged membrane environments, complementary to calmodulin, which acts in neutral and possibly positively charged lipid environments.
Poul Nissen explains:
"The activation appears to be of particular importance in the presynaptic area of neurons, which is responsible for transmitting signals in the brain's neural network. It is known that aSN accumulates in the presynaptic compartment, and our study shows, for the first time, that aSN and PMCA appear together in this area. In addition, we show that aSN stimulates calcium excretion from neurons, and not least that aSN significantly increases the activity of the PMCA."
In addition, the researchers show by mathematical network modeling that PMCA activation likely to be of key importance for the calcium balance (homeostasis), when neurons signal repeatedly, and calcium therefore enters the cell constantly. aSN's activation of PMCA thus prevents a build up of calcium that would be toxic to the cell.
Poul Henning Jensen states:
”Our laboratory has previously shown that early clump stages of aSN activate another calcium pump, SERCA, which sits in an internal organelle of the cell (endoplasmic reticulum), but SERCA is not activated much by soluble aSN. Oppositely, PMCA is activated a lot by soluble aSN and almost not by clumped aSN .”
Calcium regulation by PMCA and SERCA is essential for all cells, not least neurons, so if a pathological condition is associated with transitioning from natural aSN to clumped aSN, there is also a shift in aSN activation from PMCA to SERCA, and calcium regulation is thus strongly affected. It changes a multitude of processes, and ultimately leads to cell death.
The crucial role of calcium regulation
Calcium regulation is fundamental to the function of all cells, including neurons. It plays a crucial role in signal transmission, particularly in the presynaptic area where native aSN is typically found. When aSN clumps, this balance is therefore shifted from PMCA- to SERCA-activated calcium regulation, and this changes the calcium balance in cells. This is thought to take place early in diseases such as Parkinson's.
This newfound understanding of aSN's dual role in calcium regulation could have profound implications for unravelling the early disease processes, especially in Parkinson's disease. By identifying the shift from natural aSN activation of PMCA to clumped aSN activation of SERCA, researchers can gain insights into the mechanisms that underlie the onset and progression of neurodegenerative conditions.
Future research and therapeutic potential
As researchers continue to explore the intricate relationship between aSN and calcium pumps, it opens the door to potential diagnostic and therapeutic strategies aimed at exploring and mitigating the early disruptions in calcium regulation.
The discovery of natural aSN's role in calcium pump activation represents an important step towards understanding the complex biology of neurodegenerative diseases and how we might be able to cure them.
DANDRITE is currently building strong positions on studies of new insights into synaptic calcium signalling in both the healthy state and under Parkinson's Disease conditions. For example, new DANDRITE group leader Chao Sun has established a research programme to locate and track the individual molecular players in synapses that regulate calcium signalling. The goal is to gain a much more precise and operational understanding of early, crucial tipping points in neurodegenerative disorders and how to steer around them.
The research results were published in the international journal EMBO J.
For further information, please contact
Professor Poul Nissen
Department of Molecular Biology and Genetics & DANDRITE
Aarhus University, Denmark
Email: pn@mbg.au.dk. mobile: +4528992295
SUPPLEMENTARY INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
Study type | Experiment. The work is based on a large, international collaboration with many types of data. Aarhus: primarily from Poul Henning Jensen and Poul Nissen’s labs at aSN activation of PMCA, measured both biochemically and in cells (also with help from Lene Nejsum and Robert Edwards labs in the final stage of revision) Copenhagen: Birthe Kragelund and Annette Langkilde labs on studies of dynamic aSN interaction with PMCA measured with NMR Berlin and Aarhus: Viktor from Aarhus got good help from Jorin and Edda from Berlin on network modeling, which shows that aSN activation of PMCA is essential for a stable calcium regulation in active neurons - otherwise they build up higher and higher calcium concentration. |
External funding | This work was supported by a “Mobility plus, 3rd edition” fellowship grant of the Polish Ministry of Science and Higher Education to A.K., a postdoctoral research grant from the Extremadura Province (PO17009) to M.C.B., by a PhD stipend from the Aarhus Graduate School of Science at Aarhus University to S.T.L., by a collaborative grant from H. Lundbeck A/S to P.H.J., by Lundbeck Foundation grants R223-2015-4222 for P.H.J. and R248-2016- 2518 for Danish Research Institute of Translational Neuroscience-DANDRITE, Nordic-EMBL Partnership for Molecular to P.N and P.H.J., and by funds from a project 2 research grant from the Independent Research Fund Denmark (7014-00328B) and the Brainstruc research center (R155-2015-2666) and a professorship grant (R310-2018-3713) of the Lundbeck Foundation to P.N. Further support was given by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 101023654, awarded to E.A.N. and the Novo Nordisk Foundation (#NNF18OC0033926 to B.B.K). All NMR data were recorded at cOpenNMR, an infrastructure facility funded by the Novo Nordisk Foundation (#NNF18OC0032996). The mathematical modeling work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany ́s Excellence Strategy – The Berlin Mathematics Research Center MATH+ (EXC-2046/1, project ID: 390685689) to E.K. |
Conflicts of interest | None |
Link to scientific paper | Antoni Kowalski, Cristine Betzer, Sigrid Thirup Larsen, Emil Gregersen, Estella A. Newcombe, Montaña Caballero Bermejo, Viktor Wisniewski Bendtsen, Jorin Diemer, Christina V. Ernstsen, Shweta Jain, Alicia Espiña Bou, Annette Eva Langkilde, Lene N. Nejsum, Edda Klipp, Robert Edwards, Birthe B. Kragelund, Poul Henning Jensen, Poul Nissen Department of Molecular Biology and Genetics, Aarhus University, Aarhus, Denmark Department of Biomedicine, Aarhus University, Aarhus, Denmark Danish Research Institute of Translational Neuroscience – DANDRITE, Aarhus University, Aarhus, Denmark REPIN and Structural Biology and NMR Laboratory, Department of Biology, University of Copenhagen, Denmark Department Biochemistry and Molecular Biology and Genetics, IBMP, University of Extremadura, Badajoz, Spain Theoretical Biophysics, Humboldt-Universität zu Berlin, Berlin, Germany Department of Clinical Medicine, Aarhus University, Aarhus N, Denmark Departments of Neurology and Physiology, University of California San Francisco, San Francisco, CA. Department of Drug Design and Pharmacology, University of Copenhagen, Denmark "Monomeric α-synuclein activates the plasma membrane calcium pump" |
