The therapeutic antibody eculizumab caught in action
In collaboration with Alexion Pharmaceuticals, Inc., scientists from Aarhus University have used X-rays to understand how the therapeutic antibody eculizumab prevents our immune system from destroying red blood cells and damaging kidney tissue.
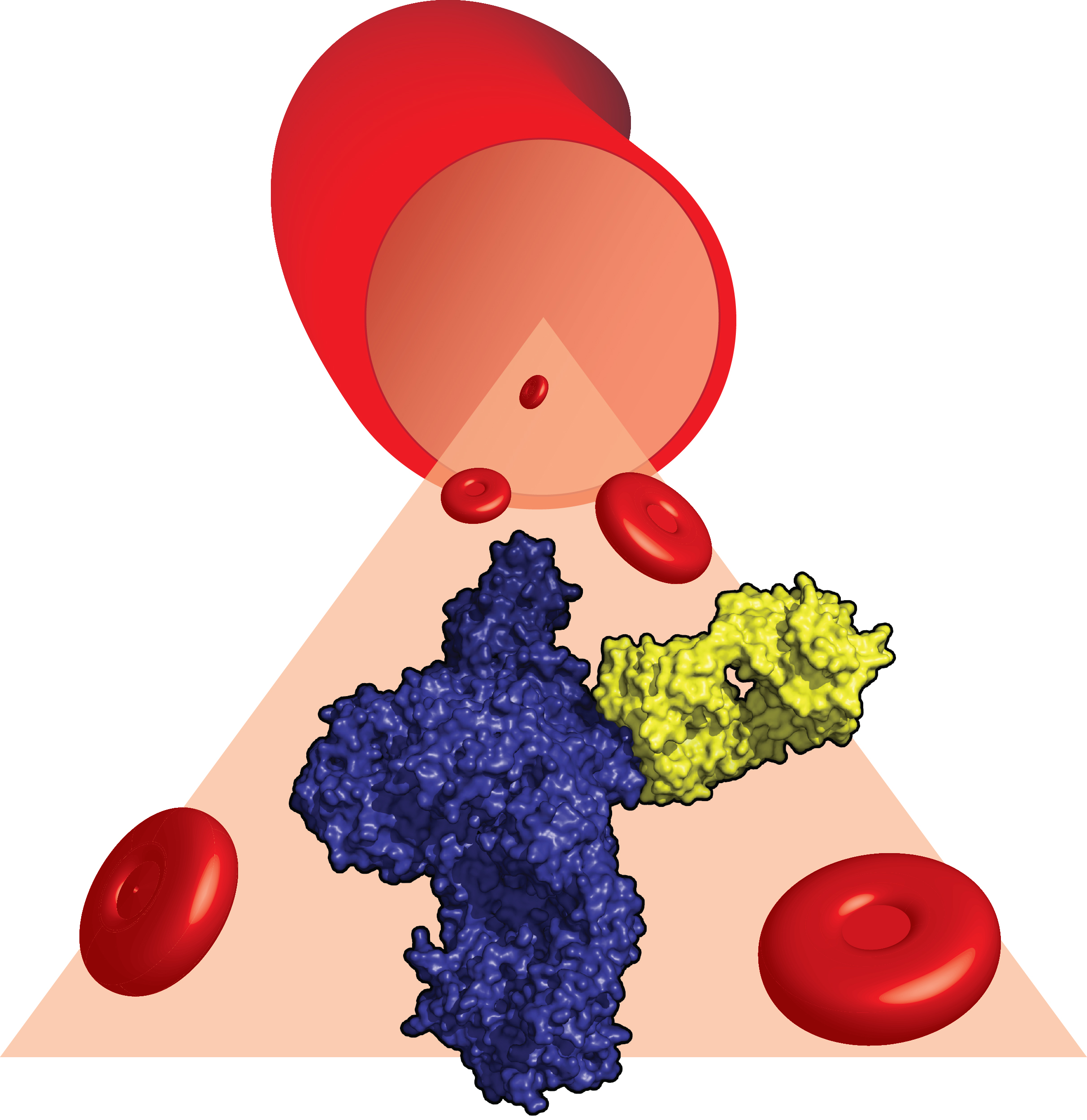
The scientists in Aarhus studied an important protein from the innate immune system called C5 which is cleaved by enzymes when pathogens invade our body as a defence mechanism. The two C5 fragments formed through this cleavage recruit immune cells to fight the pathogen and may directly kill it.
Our own cells are protected against damage caused by cleavage of the C5 protein. But in two rare diseases called paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), this protection is lost due to mutations in our DNA. Patients suffer as their red blood cells and cells in the kidney are continuously damaged, and the diseases are life-threatening. The global biopharmaceutical company Alexion, headquartered in the U.S., has developed a therapeutic antibody called eculizumab that prevents the cleavage of C5 and thereby treats the two diseases.
The research was performed by PhD student Janus Asbjørn Schatz-Jakobsen and Professor Gregers Rom Andersen at the Department of Molecular Biology and Genetics at Aarhus University in Denmark in collaboration with scientists at Alexion and is now published in The Journal of Immunology with a figure from the article on the cover of the July issue.
Description of the research project
Janus first purified C5 from human blood plasma and then mixed it with a fragment of the eculizumab antibody prepared by Alexion solely for this collaboration. After many attempts, Janus managed to form crystals containing the complex between C5 and the antibody fragment. In the following year, more than 200 crystals were investigated by Janus using very intense X-ray radiation at large international facilities in France. After a total of 18 months with careful and tedious work, Janus was finally able to describe the atomic structure of C5 bound to the eculizumab fragment. This structure revealed that the antibody creates a physical barrier that prevents the cleaving enzymes from forming contacts with C5.
On the other side of the Atlantic Ocean, scientists at Alexion in parallel performed biochemical experiments in which the function of antibodies carrying mutations in regions responsible for binding to C5 were compared to the normal eculizumab antibody.
Janus Asbjørn Schatz-Jakobsen explains: "When I had finished my atomic model and was awaiting the results from Alexion for comparison, I felt almost like a small child waiting for X-mas eve. When the result arrived by email, I could quickly confirm that there was an excellent agreement between my results and those obtained by the Alexion scientists, which confirmed that my model was correct. It was quite a relief and the results from Alexion also made me discover new exciting aspects of my atomic structure that I had not noticed yet."
The new research has also helped the scientists at Aarhus University to better understand the mechanism by which C5 is cleaved by enzymes in the absence of eculizumab. At Alexion, the atomic structure is now used as a framework to understand in details how their therapeutic antibody works and treats the two diseases.
Read the article published in The Journal of Immunology.
For further information, please contact
Professor Gregers Rom Andersen
gra@mbg.au.dk - mobile: +45 3025 6646
PhD student Janus Asbjørn Schatz-Jakobsen
janusasj@mbg.au.dk - Tel. +45 8715 4201
Department of Molecular Biology and Genetics
Aarhus University, Denmark
