MBG-forskere afdækker nye detaljer om calcium-transport i planter
Et nyt studie fra Poul Nissens forskningsgruppe viser, hvordan et calcium-transporterende protein i planters cellemembran regulerer sig selv. En dybere forståelse af, hvordan calcium-koncentration styres i planter, kan være med til at underbygge fremtidens udvikling af plantesorter med bestemte egenskaber f.eks. nye eller forbedrede afgrøder.
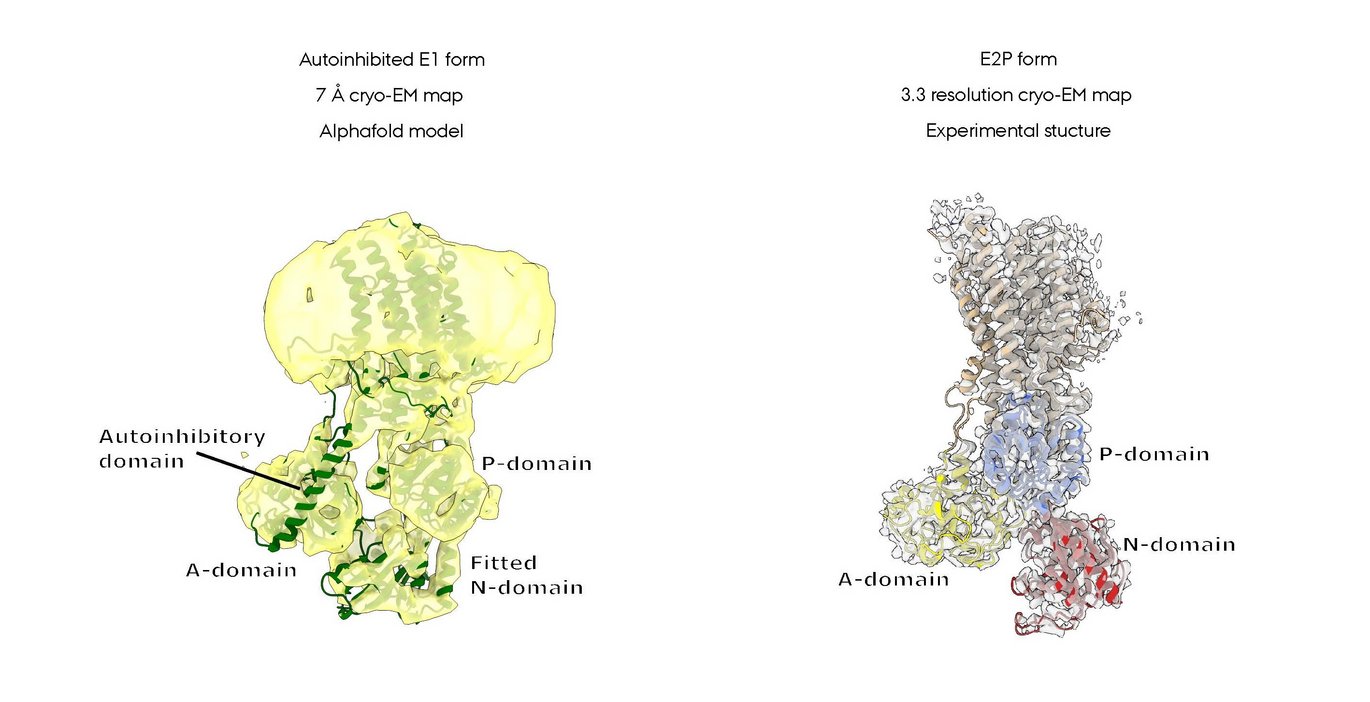
Plants use calcium signalling to control their responses to environmental conditions such as light, temperature and mechanical stress, and to the activity of other living organisms such as pathogens and symbionts. This signalling is heavily dependent on the so-called P2B calcium ATPases, large membrane-embedded Ca2+ pumps that help maintain a steep Ca2+ gradient across the cell membrane.
In a new research paper, published in Journal of Molecular Biology, Poul Nissen’s research group provides structural and functional insights into how the P2B ATPase ACA8 is auto-regulated and how this enables fine-tuning of its activity. The researchers found that a long tail of the ACA8 Calcium pump (see figure) folds in a way that prevents Ca2+ binding and transport and thus keeps the protein in an inactive state. In activation, this tail can bind two copies of the calcium-sensing protein calmodulin, and when they bind, the tail is released from the core structure of ACA8, which can then transport Ca2+ across the cell membrane.
‘This has been a very long and very complicated project’, Poul Nissen explains, ‘and I’m immensely impressed with how in particular Sigrid, Josephine and Christine managed to keep it on course over several years and, worth noting, while also giving birth to a total of seven kids. We are very proud and happy to see the paper out finally. The results will be of significant interest to a large community interested in how plants regulate their calcium level and how that affects for example plant growth – and we can also take it further to our understanding of calcium regulation in brain cells, for example.’
The key findings in the paper are based on electron microscopy (cryo-EM) structure data obtained at the national infrastructure facility EMBION at Aarhus University, combined with computational studies using the EMCC GPU cluster, and functional studies using yeast models and purified protein. The research project was carried out in Collaboration with Lisbeth Rosager Paulsen and Michael Palmgren at the University of Copenhagen.
Den videnskabelige artikel:
Conserved N-terminal Regulation of the ACA8 Calcium Pump with Two Calmodulin Binding Sites
Sigrid Thirup Larsen 1, 2, Josephine Karlsen Dannersø 1, 2, Christine Juul Fælled Nielsen 1, 2,
Lisbeth Rosager Poulsen 3, Michael Palmgren 3, Poul Nissen 1, 2
1 - Department of Molecular Biology and Genetics, Aarhus University, Aarhus, Denmark
2 - Danish Research Institute of Translational Neuroscience – DANDRITE, Aarhus University, Aarhus, Denmark
3 - Department of Plant and Environmental Sciences, Copenhagen University, Thorvaldsensvej 40, DK-1871, Denmark
Journal of Molecular Biology, Volume 436, Issue 20, 15 October 2024, 168747 https://doi.org/10.1016/j.jmb.2024.168747
SUPPLERENDE OPLYSNINGER
Vi bestræber os på, at alle vores artikler lever op til Danske Universiteters principper for god forskningskommunikation. På den baggrund er artiklen suppleret med følgende oplysninger:
Studietype:
Forskningsartikel
Ekstern finansiering:
This study was supported by Lundbeck Foundation grant no. DANDRITE-R248-2016-2518 and grant no. R310-2018-3713 as well as The Danish National Research Foundation, Center for Membrane Pumps in Cells and Disease – PUMPkin and The Independent Research Fund Denmark – Natural Sciences, grant no. 7014-00328B.
Interessekonflikt:
Ingen
Mere information
Poul Nissen
Institut for Molekylærbiologi og Genetik
Aarhus Universitet
