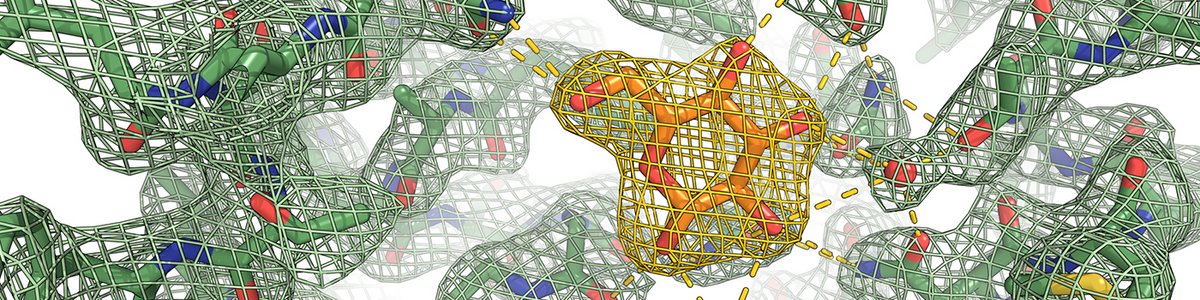
We address fundamental scientific questions pertaining to the molecular mechanism and regulation of transmembrane transporters, essential for central metabolic pathways. We are interested in proton-driven uptake of hormones, sugars and sterols in plants.
Transmembrane transport proteins are essential for a broad range of physiological processes, including growth and survival in eukaryotes. An improved understanding of the molecular underpinnings of these uptake systems has tremendous potential to address challenges in health, green transition and biodiversity.
We use a combination of structural, biochemical and biophysical approaches to characterize fundamental transporter properties. This creates a foundation to predict and augment transport properties.
We are located at the Department of Molecular Biology and Genetics at Aarhus University. The group is an international research team with diverse scientific backgrounds. We are always looking for postdocs, PhD students and Master students. If you find our work interesting and want to join us, please contact Bjørn Panyella Pedersen (also see http://pedersenlab.dk).
Group photo 2023

Our research are focused on structural and biochemical characterization of eukaryotic transporters, mainly plant transporters, as well as the methods development needed to support this characterization. In particular, we are interested in proton-driven uptake of sugars, hormones and sterols in plants.
We address these topics using a complementary set of methods founded in electron microscopy and macromolecular crystallography to determine the 3-dimensional atomic structures of key players. This is combined with biochemical characterization of the molecular mechanism in vitro and in vivo. We are engaged in development of methods for low-resolution crystallography and single-particle cryo-electron microscopy, as it pertains to the field of membrane protein structure determination.
Plant growth is the result of the integration of information from many chemical cues, but the tissue localization of the growth hormone auxin is paramount to initiate, sustain and coordinate growth. In the growing meristem of plants, auxin gradients are created by a directional cell-to-cell polar auxin transport mechanism. The local auxin gradient that this generates functions as the pattern- and organ polarity-organizing signal in plants.
The asymmetric and polar auxin gradient across cells is created by the PIN (PIN-formed) family of transporters which have a polar localization at the plasma-membrane and export auxin. Activity and regulation of PINs is the defining feature of polar auxin transport. At the protein level a number of parameters determine correct polar auxin gradients, namely specificity, affinity, and activity/inhibition.
The long-term objective of the lab is to understand the molecular mechanism and regulation of transport of auxin by determining structures, and use this information to guide our biochemical studies of mechanism.
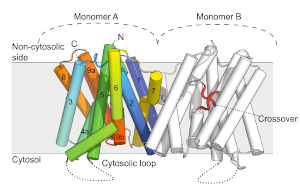
Sugars are the major cellular source of energy and carbon, and proton-driven sugar transport in plants is made possible by sugar transporters belonging to two families: the ubiquitous Sugar Porter (SP) protein family as well as the Glycoside-Pentoside-Hexuronide (GPH):cation symporter family.
In plants, Sugar Transport Proteins, from the SP family, are key determinants of monosaccharide uptake into apoplastically isolated organs. STPs control organ development, including the growth and deveopments of seeds (cereals), fruit and roots. The long-term objective of the lab is to understand the molecular mechanism and regulation of transport of sugar in the Sugar Porter family by determining structures, and use this information to guide our biochemical studies of mechanism.
Long range transport of sugars in plants happens in the phloem. Here disaccharide transporters called SUCs from the GPH family are responsible for phloem loading of sucrose, essential for generating flow of all nutrients through this vascular tissue and for broad transport of sugars from the green tissues. The long-term objective of the lab is to understand the molecular mechanism and regulation of these transporters.
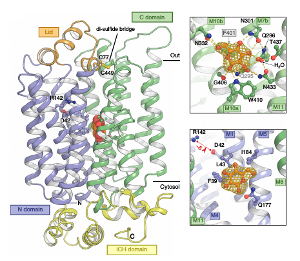
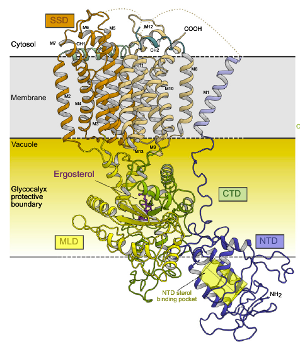
Sterols are an essential component of membranes in all eukaryotic cells and are also the precursor of multiple indispensable cellular metabolites (e.g. estrogen and testosterone in humans).
Universally, the first sterol uptake step in eukaryotes is the endocytosis of lipid particles into degradative organelles called lysosomes. Sterols are integrated into the lysosomal membrane by the Niemann-Pick type C (NPC) system and then reshuffled to other cellular membranes by vesicular and non-vesicular processes using e.g. cytosolic Lipid Transfer Proteins.
Sterol uptake and homeostasis is of high medical relevance for a number of reasons; atherosclerotic cardiovascular disease is linked to cholesterol levels in blood and Filovirus entry (eg. Ebola) into the cell is dependent on this elusive process. The NPC proteins are a highly valuable group of targets to address a range of important maladies, as well as to improve fundamental understanding of essential sterol uptake pathways for which the molecular mechanism remains almost completely unknown.
Very little is known about the molecular interactions of NPC proteins with substrates and interaction partners, including whether NPC membrane proteins mediate any kind of active transport of sterols. These questions lies at the core of understanding how sterol uptake and homeostasis is maintained and how NPC proteins confer their essential biological function in vivo. The long-term objective of the lab is to elucidate the molecular mechanisms underlying NPC-dependent sterol transport through a combination of crystallography and electron microscopy.
Bjørn Panyella Pedersen har brugt det meste af sit forskerliv på at studere molekylers bevægelser hen over cellemembraner
Da Bjørn Panyella Pedersen tilbage i gymnasiet valgte biologien, skyldtes det, at han oplevede det som det mest humanistiske naturvidenskabelige fag, der findes. Hurtigt fandt han ind i molekylærbiologien og mere specifikt den gren, der beskæftiger sig med, hvordan molekyler lykkes med at bevæge sig hen over cellemembraner og gøre nytte de rigtige steder i en organisme. I dag arbejder han konkret med at forstå, hvordan sukker, hormoner og kolesterol kommer derhen i cellen, hvor de skal være.
Podcast: https://bit.ly/podcast_panyella
YouTube: https://www.youtube.com/watch?v=PsPnW2Nwya0
Potræt: https://www.carlsbergfondet.dk/da/Nyheder/Formidling/Maanedensforsker/Bjoern-Panyella-Pedersen
Fedtstoffet kolesterol, som ellers ofte omtales som noget negativt, er livsnødvendigt for kroppens eksistens.
Men hvad bruger kroppen kolesterol til? Hvor kommer det fra? Og hvad sker der i kroppen, hvis den får for meget kolesterol?
Det handler - som med så meget andet - om balance, og for meget kolesterol er sundhedsskadeligt. Men i de rigtige mængder er kolesterol altså essentiel for en velfungerende krop, forklarer Bjørn Panyella Pedersen, som er gæst i denne uges udgave af Vidensselskabet